Abstract
Background
Both surgeon and hospital procedure volumes have been found to be associated with total hip arthroplasty (THA) outcomes. However, little research has been conducted on the relative influence. We studied the association between THA survivorship and both hospital and surgeon procedure volumes, considering their relative impact.
Methods
A population-based cohort included all patients aged ≥40 years having received a unilateral primary THA from 2010 to 2011, from the French National Health Insurance Database. Patients were followed up until the end of 2014. The outcome was THA revision. Exposures of interest were procedure volumes, divided into tertiles: <1.5, 1.5-4, >4 and <7, 7-15, >15 procedures per month defined as low, medium, and high volumes for surgeon and hospital, respectively.
Results
The cohort had 62,906 patients, with mean age 69 years and women 57%. Mean surgeon and hospital volumes were 8 and 23 procedures per month, respectively, and 5%, 72%, 22% and 7%, 28%, 65% of THAs were implanted by a low-, medium-, and high-volume surgeon or in a low-, medium-, and high-volume hospital, respectively. Median follow-up was 45 months (range, 0-57 months). In multivariate analysis, adjusted for both surgeon and hospital volumes, for patient and THA characteristics, a lower surgeon volume was associated with poorer THA survivorship (adjusted hazard ratio [aHR] = 1.19; 95% confidence interval [CI], 1.07-1.34 and aHR = 1.70; 95% CI, 1.40-2.05, for medium- and low-volume surgeon, respectively, compared with that of high volume), whereas hospital volume was not.
Conclusions
This study brings evidence to support the notion that THAs performed by high-volume surgeons in French private hospitals have higher survivorship in the first 4 years.
Keywords: Total hip arthroplasty survivorship, Surgeon activity volume, Hospital activity volume
Introduction
In previous studies, both surgeon and hospital procedure volumes have been found to be associated with surgery outcomes, across quite a wide range of procedures and conditions [1], [2], [3], [4]. In the case of primary total hip arthroplasty (THA), low surgeon volume has been found to be associated with higher dislocation rates [5], [6], more blood loss, postoperative complications [6], higher rates of deep wound infection [7] within the months after surgery, and higher revision rates after 1 year [8], 2 years [9], and 33 months [10]. Low hospital volume has been found to be associated with longer lengths of stay (LOSs) [11], higher dislocation rates during the stay [12], and higher revision rates [13], [14], [15].
Most studies on the subject have examined the influence of either surgeon or hospital procedure volume on those outcomes. However, few researchers have studied the relative influence of both factors after adjusting for the other and have shown that the impact of hospital procedure volume remains unclear: the association between revision rates and hospital procedure volume was no longer significant when the surgeon procedure volume was also considered [6], [8], [10], [16], [17].
However, the vast majority of these studies were conducted in North America or Japan, the only one on European data used Nordic registers [18], and none were conducted in France. Now, since the association between hospital procedure volume and outcomes may vary significantly depending on the health care system, there are limitations to extrapolating the aforementioned results to countries with different health care systems and surgeon training systems.
The aim of our study was to assess the impact of both hospital and surgeon procedure volumes on THA survivorship at 4 years follow-up in a large French nationwide cohort.
Material and methods
Data sources
We retrospectively used the French National Health Insurance Information System (Système National d'Informations Inter-régimes de l'Assurance Maladie [SNIIRAM]), from which relevance and accuracy for pharmacoepidemiology studies have been validated [19], [20], [21], [22], [23], [24] and used in many published studies [13], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. The French National Health Insurance is compulsory; it comprehensively covers the entire French population. The only possible depletion from the SNIIRAM is for the subjects who leave France, do not use anymore any health services, and do not receive any reimbursement for medicines or medical procedures. This is the reason why we excluded 767 (0.2%) patients, who did not receive any reimbursement 6 months after THA, for they were not in the SNIIRAM anymore after their THA (probably patients come to France to receive their THA and having left after hospital discharge).
The SNIIRAM is divided into 3 main schemes depending on the professional position: (1) general scheme for employees in industry and business, (2) agricultural scheme covering farmers and farm employees, and (3) social scheme for independent workers. In our study, only the general scheme beneficiaries have been included (approximately 77% of the population) because of technical reasons: for beneficiaries of other schemes, some information about medical details, long-term disease, or date of death do not follow the same recording process into the databases and are available partially or with long delays. For beneficiaries of the general scheme, the SNIIRAM provides comprehensive records—including dates—on prescribed outpatient drugs (Anatomical Therapeutic Chemical classification codes) and medical devices, and reimbursed services and procedures. The database does not stipulate the medical indication for each reimbursement, the surgical approach, or the side (left vs right), but it does contain the patients' demographic, administrative and medical details (including any long-term conditions, such as diabetes mellitus, cancer, or cardiovascular disease), and the date of their death.
In addition, an anonymous unique identifier for each patient links SNIIRAM information to the national hospital discharge database (Programme de médicalisation des systèmes d'information [PMSI]), which covers all hospitals and provides reasons for admission (in the International Classification of Diseases, Tenth Revision, format). As performed and validated in previous studies [13], [21], [22], [32], [35], we used both procedures and medical devices reimbursed from hospital claims, to capture patients with primary THA. We had 19,564 (3.8%) patients with incoherent data in the PMSI, and 5639 (1.7%) THAs with missing characteristics; we excluded them. Since the payment method for surgeons working in public hospitals is not fee-for-service, unlike surgeons working in private hospitals, the surgeon is not identified in the public hospital databases but is recorded in private hospital databases; we therefore limited our study to private hospitals.
Approval was obtained from the French Data Protection agency (Commission Nationale de l'Informatique et des Libertés). Informed consent was not required as information was collected anonymously.
Study population
A population-based cohort was created from the French National Health Insurance database and included all patients aged ≥40 years having been implanted with a primary THA for a reason other than trauma or bone cancer from April 1, 2010, to December 31, 2011, and who were covered by the general Health Insurance scheme.
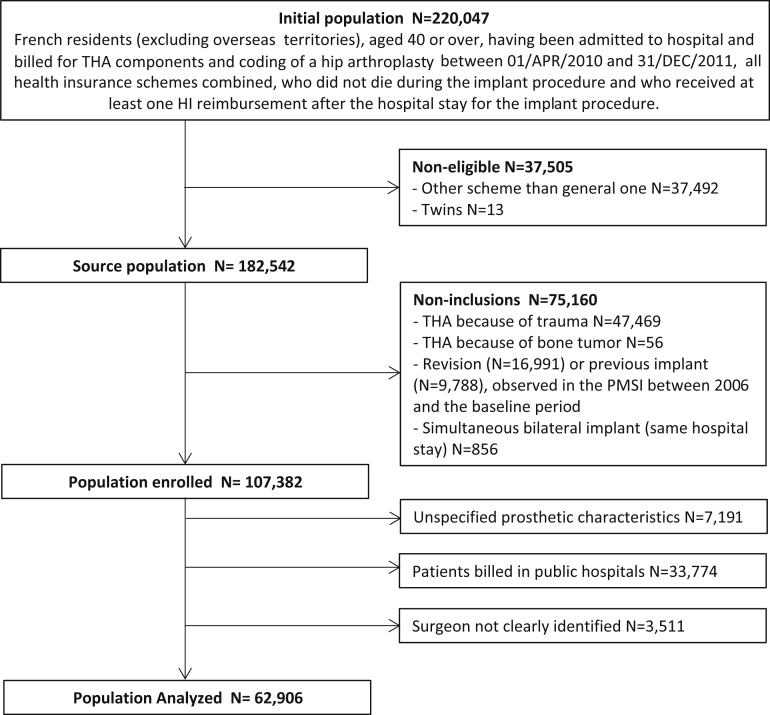
Patients having received primary THA prosthetic revision before the index date, simultaneous bilateral THA, not having received any reimbursement 6 months after THA (therefore impossible to follow-up), or with incoherent data in PMSI were excluded. From this initial cohort of 100,191 implanted patients, we excluded all patients operated on in public hospitals (N = 33,774; 33.7%), and those for whom the primary surgeon could not be precisely identified (several surgeons were involved; N = 3511). The final sample for this analysis, therefore, included 62,906 THAs (Fig. 1).
Figure 1.
Study population flowchart. HI, Health Insurance.
Variables of interest: hospital volume and surgeon volume
Procedure volumes for both surgeon and hospital were calculated annually during the administrative year the THA was performed. Only primary THAs were used in the calculation, not revisions. Hospital and surgeon volumes were then divided into thirds of the distribution, giving case volume groupings close to those published in the literature [8], [10], [12]. The hospital volume groups compared were as follows: “<7 procedures per month,” “7-15 procedures per month,” and “>15 procedures per month,” defining “low-,” “medium-,” and “high-activity” hospital groups, respectively. The surgeon volume groups compared were as follows: “<1.5 procedures per month,” “1.5-4 procedures per month,” and “>4 procedures per month,” similarly defining “low-,” “medium-,” and “high-activity” surgeon groups, respectively.
Covariates
We adjusted for a series of patient and implantation center characteristics previously shown to be associated [15], [36], [37] with a risk of complications after joint arthroplasty. The patients' sociodemographic variables, namely age, gender, and social deprivation index [38], as well as patients' medications to adjust for were obtained from the SNIIRAM database; medications were identified with prescriptions (Anatomical Therapeutic Chemical classification codes) reimbursed at least once within 90 days of inclusion, namely, benzodiazepines, antihypertensive agents, osteoporosis treatments, oral corticosteroids, lipid-lowering agents, or antidepressants. Regarding medical conditions, diabetes and morbid obesity (comorbidities to adjust for) were obtained from both the SNIIRAM and PMSI: they were defined (the International Classification of Diseases, Tenth Revision, categories) on the basis of hospital discharge reports or a long-term condition recorded during the year before inclusion together with relevant prescriptions [13].
If ever a patient was dead during the study period, the date of death was also registered in the SNIIRAM database. The duration of the hospital stay (LOS, in days) was also obtained from the hospital discharge database.
Continuous data (age, social deprivation index, and LOS) were divided into categories. Age groups were “40-59,” “60-74,” and “≥75 years.” LOS was divided into 3 groups: “8-9 days,” corresponding to the “standard LOS” (the most frequent, as well as mean and median LOS in private hospitals for primary THA in France and Europe [39], [40], [41]), “<8 days” for stays shorter than the “standard LOS” (corresponding to patients with immediate recovery being better/shorter than the “average patient” receiving THA), and “>9 days” for stays longer than the “standard LOS” (corresponding to patients with immediate recovery being longer than the average patient). Deprivation index was divided into quintiles [38].
The fixation technique groups were uncemented, antibiotic-free cement, and antibiotic-impregnated cement [13]. Four bearing couples were analyzed: ceramic-on-ceramic, ceramic-on-polyethylene, metal-on-metal, and metal-on-polyethylene.
Outcome
The outcome was THA revision (any surgical reintervention in which the implant or any of its components was changed or removed). Observations were right censored on December 31, 2014, or at the last date of health services use, if neither revision nor death had yet occurred.
Statistical analysis
Categorical variables were described using frequency (percentage). Univariate analyses were performed using the Pearson chi-square test for percentage comparisons, and univariate Cox models and log-rank tests for survivorship data comparisons (providing hazard ratios [HRs]). A multivariate analysis was performed using a competing risk Fine and Gray regression model with death as a competing risk to provide adjusted HRs (aHR).
Complementary analyses were also performed with multilevel models, to take into account the hierarchical structure of the data (patients being implanted by the same surgeon and surgeons working in the same hospital forming clusters). In the first complementary analysis, we used 2 Cox frailty models to consider correlations between failures within the same cluster: in the first model, we considered the clustering at hospital level, and in the second, clustering was at surgeon level. In a second complementary analysis, we used a hierarchical logistic regression, taking into account both surgeon and hospital levels in the same model, with a surgeon random effect nested within a hospital random effect; in this analysis, a binary outcome (prosthesis revised or not, at 5 years follow-up) was used instead of a survival outcome.
Statistical analyses were conducted with R.1.0 (The R Foundation for Statistical Computing) and SAS 9.3 software (SAS Institute Inc., Cary, NC). All tests were 2 sided with a 0.05 alpha risk.
Results
Baseline characteristics of the cohort
Mean (standard deviation [SD]) age was 69 (11) years and 57% of the individuals were women. Fixation was uncemented in 76% and the THA bearing surface was ceramic-on-ceramic in 46%. Mean (SD) LOS was 9 (3) days. Mean (SD) hospital volume was 23 (14) procedures per month; 7%, 28%, and 65% of THAs were implanted in a low-, medium-, and high-activity hospital, respectively. Mean (SD) surgeon volume was 8 (6) procedures per month; 5%, 72%, and 22% of THAs were implanted by a low-, medium-, and a high-activity surgeon, respectively (Table 1).
Table 1.
Characteristics of total study sample and according to hospital and surgeon volume groups.
| Covariates | Whole sample (N = 62,906) | Hospital volume of procedures |
Surgeon volume of procedures |
||||
|---|---|---|---|---|---|---|---|
| <7 per mo (N = 4467) | 7-15 per mo (N = 17,399) | >15 per mo (N = 41,040) | <1.5 per mo (N = 3417) | 1.5-4 per mo (N = 45,348) | >4 per mo (N = 14,141) | ||
| Patients | |||||||
| Gender, n (%) | |||||||
| Men | 26,861 (43) | 1929 (43) | 7321 (42) | 17,611 (43) | 1459 (43) | 19,451 (43) | 5951 (42) |
| Women | 36,045 (57) | 2538 (57) | 10,078 (58) | 23,429 (57) | 1958 (57) | 25,897 (57) | 8190 (58) |
| Mean age (std error), y | 69 (11) | ||||||
| Age category, n (%) | |||||||
| 40-60 y | 11,677 (19) | 803 (18) | 3069 (18) | 7805 (19) | 703 (21) | 8330 (18) | 2644 (19) |
| 60-75 y | 28,565 (45) | 1858 (42) | 7840 (45) | 18,867 (46) | 1456 (43) | 20,436 (45) | 6673 (47) |
| ≥75 y | 22,664 (36) | 1806 (40) | 6490 (37) | 14,368 (35) | 1258 (37) | 16,582 (37) | 4824 (34) |
| Deprivation index, n (%) | |||||||
| 1 | 10,617 (17) | 742 (17) | 3197 (18) | 6678 (16) | 831 (24) | 7462 (16) | 2324 (16) |
| 2 | 11,510 (18) | 833 (19) | 2973 (17) | 7704 (19) | 667 (20) | 8283 (18) | 2560 (18) |
| 3 | 11,662 (19) | 888 (20) | 2596 (15) | 8178 (20) | 498 (15) | 8399 (19) | 2765 (20) |
| 4 | 11,947 (19) | 748 (17) | 3443 (20) | 7756 (19) | 542 (16) | 8855 (20) | 2550 (18) |
| 5 | 12,388 (20) | 685 (15) | 3965 (23) | 7738 (19) | 566 (17) | 8948 (20) | 2874 (20) |
| Missing | 4782 (8) | 571 (13) | 1225 (7) | 2986 (7) | 313 (9) | 3401 (7) | 1068 (8) |
| Diabetes, n (%) | 6925 (11) | 590 (13) | 2057 (12) | 4278 (10) | 405 (12) | 5128 (11) | 1392 (10) |
| Morbid obesity, n (%) | 105 (0) | ||||||
| Benzodiazepines, n (%) | 23,988 (38) | 1691 (38) | 6742 (39) | 15,555 (38) | 1419 (42) | 17,411 (38) | 5158 (36) |
| Antihypertensives, n (%) | 37,130 (59) | 2691 (60) | 10,619 (61) | 23,820 (58) | 1990 (58) | 26,998 (60) | 8142 (58) |
| Antidepressants, n (%) | 9259 (15) | 704 (16) | 2614 (15) | 5941 (14) | 560 (16) | 6775 (15) | 1924 (14) |
| Antiosteoporotics, n (%) | 5376 (9) | 429 (10) | 1492 (9) | 3455 (8) | 311 (9) | 3881 (9) | 1184 (8) |
| Steroids, n (%) | 9934 (16) | 757 (17) | 2801 (16) | 6376 (16) | 588 (17) | 7262 (16) | 2084 (15) |
| Lipid-lowering medication, n (%) | 24,420 (39) | 1734 (39) | 6976 (40) | 15,710 (38) | 1279 (37) | 17,729 (39) | 5412 (38) |
| Prosthesis | |||||||
| Bearing surface, n (%) | |||||||
| MoP | 18,601 (30) | 1716 (38) | 5441 (31) | 11,444 (28) | 1141 (33) | 13,955 (31) | 3505 (25) |
| CoC | 28,721 (46) | 1712 (38) | 7380 (42) | 19,629 (48) | 1524 (45) | 19,918 (44) | 7279 (51) |
| CoP | 13,936 (22) | 984 (22) | 4093 (24) | 8859 (22) | 707 (21) | 10,094 (22) | 3135 (22) |
| MoM | 1648 (3) | 55 (1) | 485 (3) | 1108 (3) | 45 (1) | 1381 (3) | 222 (2) |
| Fixation type, n (%) | |||||||
| No cement | 47,734 (76) | 3552 (80) | 13,420 (77) | 30,762 (75) | 2510 (73) | 34,955 (77) | 10,269 (73) |
| No antibiotics | 2063 (3) | 217 (5) | 441 (3) | 1405 (3) | 104 (3) | 1523 (3) | 436 (3) |
| Antibiotics | 13,109 (21) | 698 (16) | 3538 (20) | 8873 (22) | 803 (24) | 8870 (20) | 3436 (24) |
| Hospital stays | |||||||
| Mean length of stay (std error), d | 8.6 (2.9) | ||||||
| Length of stay category, n (%) | |||||||
| <8 d | 21,952 (35) | 953 (21) | 5034 (29) | 15,965 (39) | 853 (25) | 14,296 (32) | 6803 (48) |
| 8-9 d | 26,659 (42) | 1812 (41) | 7227 (42) | 17,620 (43) | 1410 (41) | 19,817 (44) | 5432 (38) |
| >9 d | 14,295 (23) | 1702 (38) | 5138 (30) | 7455 (18) | 1154 (34) | 11,235 (25) | 1906 (13) |
| Median hospital volume (interquartile range), acts/mo | 12 (6-19) | ||||||
| Hospital volume category, n (%) | |||||||
| <7 per mo | 4467 (7) | 984 (29) | 3469 (8) | 14 (0) | |||
| 7-15 per mo | 17,399 (28) | 1345 (39) | 15,602 (34) | 452 (3) | |||
| >15 per mo | 41,040 (65) | 1088 (32) | 26,277 (58) | 13,675 (97) | |||
| Median surgeon volume (interquartile range), acts/mo | 2.6 (1.1-5.2) | ||||||
| Surgeon volume category, n (%) | |||||||
| <1.5 per mo | 3417 (5) | 984 (22) | 1345 (8) | 1088 (3) | |||
| 1.5-4 per mo | 45,348 (72) | 3469 (78) | 15,602 (90) | 26,277 (64) | |||
| >4 per mo | 14,141 (22) | 14 (0) | 452 (3) | 13,675 (33) | |||
CoP, ceramic-on-polyethylene; MoM, metal-on-metal; MoP, metal-on-polyethylene; std, standard.
Characteristics of the cohort according to hospital and surgeon volumes
In univariate analyses, all covariates were significantly associated to hospital and to surgeon procedure volumes, except for gender, benzodiazepines, hospital procedure volume and antiosteoporotics, and surgeon procedure volume (Table 1).
It is also notable that hospital and surgeon procedure volumes were correlated (correlation coefficient, r = 0.49; P < .001), as high-volume surgeons are more likely to work in high-volume hospitals: 97% of individuals who were implanted by high-volume surgeons were billed in high-volume centers, 3% in medium-volume and 0% in low-volume centers.
Associations between revision and hospital and surgeon volumes
Median follow-up was 1380 days (45.3 months; interquartile interval, 39.1-50.3 months); 2276 patients had a THA revision, among which 155 were revisions for implant failure or periprosthetic fracture, 357 for dislocation, 208 for infection, 1442 for mechanical complication (including aseptic loosening, osteolysis, corrosion, and adverse tissue reactions), and 114 were revisions for unspecified causes.
In the univariate analysis, hospital and surgeon procedure volumes are both associated with prosthetic survivorship (HR, 1.19; 95% CI, 1.08-1.30 for THA revision; HR, 1.29, 95% CI, 1.11-1.50 for hospitals; and HR, 1.27; 95% CI, 1.14-1.42 and HR, 1.87, 95% CI, 1.58-2.23 for surgeons, comparing medium and low volume, respectively, with high volume; Table 2).
Table 2.
Association between patients', THAs', and stays' characteristic and prosthetic revision.
| Covariates | aHR (95% CI),a N = 62,906 | P value |
|---|---|---|
| Gender | .007 | |
| Men | Ref | |
| Women | 0.90 (0.82-0.98) | |
| Age (y) | <.0001 | |
| 40-60 | 1.19 (1.07-1.33) | |
| 60-75 | Ref | |
| ≥75 | 0.86 (0.78-0.96) | |
| Diabetes | 1.11 (0.97-1.26) | .10 |
| Benzodiazepines | 1.21 (1.10-1.32) | <.0001 |
| Antihypertensives | 1.00 (0.92-1.10) | .83 |
| Antidepressants | 1.35 (1.21-1.51) | <.0001 |
| Antiosteoporotics | 1.15 (0.99-1.33) | .06 |
| Oral corticosteroids | 1.34 (1.21-1.49) | <.0001 |
| Lipid-lowering medication | 0.94 (0.85-1.02) | .11 |
| Deprivation index | .07 | |
| 1 (most fortunate) | Ref | |
| 2 | 1.01 (0.88-1.17) | |
| 3 | 1.12 (0.97-1.29) | |
| 4 | 1.19 (1.03-1.37) | |
| 5 (most deprived) | 1.15 (1.00-1.32) | |
| Missing | 1.04 (0.86-1.25) | |
| Bearing surface | .84 | |
| Metal-on-polyethylene | Ref | |
| Ceramic-on-polyethylene | 0.96 (0.85-1.09) | |
| Ceramic-on-ceramic | 1.01 (0.90-1.13) | |
| Metal-on-metal | 0.94 (0.72-1.23) | |
| Fixation type | <.001 | |
| No cement | Ref | |
| Cement without antibiotics | 0.93 (0.74-1.18) | |
| Cement with antibiotics | 0.79 (0.70-0.88) | |
| Length of stay | .05 | |
| <8 d | 1.05 (0.95-1.15) | |
| 8-9 d | Ref | |
| >9 d | 1.13 (1.02-1.26) | |
| Hospital volume (procedures/mo) | .18 | |
| <7 | 1.10 (0.93-1.29) | |
| 7-15 | 1.09 (0.99-1.20) | |
| >15 | Ref | |
| Surgeon volume (procedures/mo) | <.0001 | |
| <1.5 | 1.70 (1.40-2.05) | |
| 1.5-4 | 1.19 (1.07-1.34) | |
| >4 | Ref |
Ref, reference.
Adjusted for all factors.
In the full model, adjusted for both surgeon and hospital volumes, as well as all the covariates, surgeon volume remains associated with prosthetic revision: the aHR was 1.19, 95% CI, 1.07-1.34 and HR was 1.70, 95% CI, 1.40-2.05, for medium and low volume, respectively, compared with high volume; hospital volume is no longer associated with prosthetic revision, with the aHR being 1.09, 95% CI, 0.99-1.20 and HR being 1.10, 95% CI, 0.93-1.29 for medium and low volume, respectively, compared with high volume (Table 2).
Associations with other covariates
Age and gender are associated with prosthetic revision both in univariate and multivariate analyses, with female and older patients having a better prosthetic prognosis than men and younger patients (aHR = 0.90; 95% CI, 0.82-0.98 and aHR [40-60 vs 60-75] = 0.86; 95% CI, 0.78-0.96, respectively). Benzodiazepine, antidepressants, and oral corticosteroid consumptions are also associated with prosthetic revision (aHR = 1.21, 95% CI, 1.10-1.32; aHR = 1.35, 95% CI, 1.21-1.51; and aHR = 1.34, 95% CI, 1.21-1.49, respectively). THAs with antibiotic-impregnated cement have a better prosthetic prognosis than uncemented ones within 45 months (aHR = 0.79; 95% CI, 0.70-0.88).
Complementary analyses
The complementary analyses, taking into account the hierarchical structure of the data, either with a frailty Cox model or with a 3-level logistic regression, did not change the conclusions (Table 3).
Table 3.
Complementary analyses with multilevel models.
| Model | Volumes | Number of procedures per month | aHRa (95% CI), adjusted ORb (95% CI) | P value |
|---|---|---|---|---|
| Cox model with shared frailty at hospital level | Hospital volume | .17 | ||
| >15 | Ref | |||
| 7-15 | 1.09 (0.97-1.23) | |||
| <7 | 1.12 (0.94-1.33) | |||
| Surgeon volume | ||||
| >4 | Ref | |||
| 1.5-4 | 1.25 (1.10-1.43) | <.0001 | ||
| <1.5 | 1.75 (1.44-2.13) | |||
| Cox model with shared frailty at surgeon level | Hospital volume | .23 | ||
| >15 | Ref | |||
| 7-15 | 1.08 (0.97-1.20) | |||
| <7 | 1.10 (0.93-1.30) | |||
| Surgeon volume | <.0001 | |||
| >4 | Ref | |||
| 1.5-4 | 1.23 (1.07-1.41) | |||
| <1.5 | 1.74 (1.42-2.13) | |||
| Multilevel logistic regression (3 levels: with surgeon random effect nested within a hospital random effect) | Hospital volume | .39 | ||
| >15 | Ref | |||
| 7-15 | 1.07 (0.95-1.22) | |||
| <7 | 1.11 (0.92-1.34) | |||
| Surgeon volume | <.0001 | |||
| >4 | Ref | |||
| 1.5-4 | 1.25 (1.06-1.47) | |||
| <1.5 | 1.73 (1.38-2.17) |
OR, odds ratio; Ref, reference.
Adjusted HR, from multivariate frailty Cox model, adjusted for the same covariates as in the main analysis.
Adjusted OR, from multivariate multilevel logistic regression, adjusted for the same covariates as in the main analysis.
Discussion
Main results
In patients operated for primary THA in private hospitals in France, surgeon volume is the factor with the highest size effect in the multivariate analysis, with low surgeon volume being a risk factor of revision (aHR = 1.70; 95% CI, 1.40-2.05) compared with high surgeon volume. After adjusting for confounding factors, hospital volume had no significant impact on THA survivorship.
We observed similar results when taking the hierarchical structure of the data into account.
In French private hospitals, surgeon and hospital procedure volumes for THAs are correlated, assuming that part of the hospital volume is indeed only an indication of surgeon volume. Hospital volume may be a proxy of surgeon volume when the latter cannot be individually measured.
Consistency with the literature
Our results, at a median follow-up of 45 months, are consistent with 2 previous Canadian studies from smaller cohorts: Paterson et al [8] have shown that at 1 year follow-up, surgeon volume is associated with revision and hospital volume becomes nonsignificant in the multivariate analysis on 20,290 patients. Kreder et al [10] observed the same within 33 months’ follow-up on 3645 patients. Consistent with this, another study assessing hip dislocation within 90 days after THA on 5211 patients showed that hospital volume had no significant impact after adjusting for surgeon volume [7]. On the other hand, some studies, with a longer follow-up after a primary THA, but with far fewer patients showed different findings: at 8 years follow-up, Manley et al [42] found no significant association between THA revision and both hospital and surgeon procedure volume; the same results can be seen from the study by Losina et al [43] who found a significant association between surgeon volume and revision from 1 to 18 months, but no significant association from 19 to 48 months after a primary THA; 3 studies from the Kaiser Permanente Total Joint Replacement Registry data did not show surgeon volume as impactful, when analyzing revisions for infection [17], aseptic loosening [16], and dislocation [44]. These 5 studies were conducted in the United States, and the association between hospital procedure volume and outcomes may vary depending on the health care system and medical practices, such as LOS, which is longer in Europe than in the United States [45].
Other factors associated with prosthesis revision are antibiotic-impregnated cement, younger age [36], and consumption of benzodiazepine, antidepressants, and steroids. As regard to antibiotic-impregnated cement being associated with longer prosthetic survivorship, this is consistent with meta-analyses performed by Morshed et al [46] and Parzivi et al [47].
Regarding deprivation index, a study conducted by Agabiti et al [48] in Italy found that low income had no effect on either orthopaedic complications within 90 days or revision. Further studies should explore the relationship between deprivation and prosthetic survivorship, to gain a better understanding of the underlying mechanism that leads to poorer prosthetic survivorship in more deprived populations, found in the present study. Indeed, the impact of social condition may vary according to social support available, which can vary considerably.
Strengths and limitations
In the present study, based on the National Health Insurance Database, data were only available for patients operated on in private hospitals from April 1, 2010, to December 31, 2011. However, in France, >65% of THAs are performed in private hospitals [13].
There could be a concern that the selected patients operated on in private hospitals could be different from patients operated on in public hospitals. However, the initial characteristics of patients operated on in private and in public hospitals present no major difference for the covariates we collected (Table A of the Supplementary Material).
Despite the large number of covariates collected for adjustment in the multivariate Cox model, residual confounding because of factors not collected in our study (for being absent from the database, such as smoking history, alcohol consumption, regarding the patient or surgical approach, and head size of the implant) cannot be evaluated.
We excluded 3511 individuals (5.3%) from the analyses since the surgeon could not be identified. Nevertheless, there is no obvious reason to think these missing data would be linked to hospital or surgeon volume; therefore, we do not believe that excluding these patients had a substantial effect on the observed associations.
Conclusions
After a median of 45 months follow-up of primary THAs on a population-based cohort implanted in French private hospitals, we observed that medium- to short-term prosthetic survivorship was associated with surgeon volume but not with hospital procedure volume after adjusting for other THA revision risk factors: THAs performed by high-activity surgeons have a better prognosis. Although the existence of a minimum acceptable volume or threshold remains unproven, this study brings evidence in support of the notion that THAs performed by high-volume surgeons operating in the private system in France have higher survivorship in the first 4 years of follow-up.
Acknowledgments
S. C. and C. L. C. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S. C. and M. Z. were involved in study concept and design. C. L. C., S. C., and M. Z. were involved in the acquisition, analysis, or interpretation of data. C. L. C. and S. C. drafted the article. C. L. C., S. C., and M. Z. were involved in the critical revision of the article for important intellectual content. C. L. C. and S. C. were involved in the statistical analysis and the study supervision was done by M. Z.
Footnotes
No author associated with this paper has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2017.03.010.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.artd.2017.03.010.
Appendix A. Supplementary data
References
- 1.Shackley P., Slack R., Booth A., Michaels J. Is there a positive volume-outcome relationship in peripheral vascular surgery? Results of a systematic review. Eur J Vasc Endovasc Surg. 2000;20(4):326. doi: 10.1053/ejvs.2000.1188. [DOI] [PubMed] [Google Scholar]
- 2.Pettit S.J., Jhund P.S., Hawkins N.M. How small is too small? A systematic review of center volume and outcome after cardiac transplantation. Circ Cardiovasc Qual Outcomes. 2012;5(6):783. doi: 10.1161/CIRCOUTCOMES.112.966630. [DOI] [PubMed] [Google Scholar]
- 3.Lenzi J., Lombardi R., Gori D. Impact of procedure volumes and focused practice on short-term outcomes of elective and urgent colon cancer resection in Italy. PLoS One. 2013;8(5):e64245. doi: 10.1371/journal.pone.0064245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijboer A., Ulrich F., Bechstein W.O., Schnitzbauer A.A. Volume and outcome relation in German liver transplant centers: what lessons can be learned? Transplant Res. 2014;3(1):5. doi: 10.1186/2047-1440-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz J.N., Losina E., Barrett J. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83-A(11):1622. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Yasunaga H., Tsuchiya K., Matsuyama Y., Ohe K. High-volume surgeons in regard to reductions in operating time, blood loss, and postoperative complications for total hip arthroplasty. J Orthop Sci. 2009;14:3. doi: 10.1007/s00776-008-1289-4. [DOI] [PubMed] [Google Scholar]
- 7.Solomon D.H., Losina E., Baron J.A. Contribution of hospital characteristics to the volume-outcome relationship: dislocation and infection following total hip replacement surgery. Arthritis Rheum. 2002;46(9):2436. doi: 10.1002/art.10478. [DOI] [PubMed] [Google Scholar]
- 8.Paterson J.M., Williams J.I., Kreder H.J. Provider volumes and early outcomes of primary total joint replacement in Ontario. Can J Surg. 2010;53:175. [PMC free article] [PubMed] [Google Scholar]
- 9.Ravi B., Jenkinson R., Austin P.C. Relation between surgeon volume and risk of complications after total hip arthroplasty: propensity score matched cohort study. BMJ. 2014;348:g3284. doi: 10.1136/bmj.g3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreder H.J., Williams J.I., Jaglal S. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431. [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuyasu S., Hagihara A., Horiguchi H., Nobutomo K. Relationship between total arthroplasty case volume and patient outcome in an acute care payment system in Japan. J Arthroplasty. 2006;21:656. doi: 10.1016/j.arth.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko T., Hirakawa K., Fushimi K. Relationship between peri-operative outcomes and hospital surgical volume of total hip arthroplasty in Japan. Health Policy. 2014;117(1):48. doi: 10.1016/j.healthpol.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Colas S., Collin C., Piriou P., Zureik M. Association between total hip replacement characteristics and 3-year prosthetic survivorship: a population-based study. JAMA Surg. 2015;150(10):979. doi: 10.1001/jamasurg.2015.1325. [DOI] [PubMed] [Google Scholar]
- 14.Judge A., Chard J., Learmonth I., Dieppe P. The effects of surgical volumes and training centre status on outcomes following total joint replacement: analysis of the Hospital Episode Statistics for England. J Public Health (Oxf) 2006;28(2):116. doi: 10.1093/pubmed/fdl003. [DOI] [PubMed] [Google Scholar]
- 15.Dy C., Bozic K., Pan T.J. Risk factors for early revision after total hip arthroplasty. Arthritis Care Res. 2014;66(6):907. doi: 10.1002/acr.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khatod M., Cafri G., Namba R.S., Inacio M.C.S., Paxton E.W. Risk factors for total hip arthroplasty aseptic revision. J Arthroplasty. 2014;29(7):1412. doi: 10.1016/j.arth.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Namba R.S., Inacio M.C., Paxton E.W. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg Br. 2012;94(10):1330. doi: 10.1302/0301-620X.94B10.29184. [DOI] [PubMed] [Google Scholar]
- 18.Glassou E.N., Hansen T.B., Mäkelä K. Association between hospital procedure volume and risk of revision after total hip arthroplasty: a population-based study within the Nordic Arthroplasty Register Association database. Osteoarthritis Cartilage. 2016;24(3):419. doi: 10.1016/j.joca.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Latry K., Bégaud B. Pharmacoepidemiological research using French reimbursement databases: yes we can! Pharmacoepidemiol Drug Saf. 2010;19(3):256. doi: 10.1002/pds.1912. [DOI] [PubMed] [Google Scholar]
- 20.Bernard M.A., Bénichou J., Blin P., CADEUS Team Use of health insurance claim patterns to identify patients using nonsteroidal anti-inflammatory drugs for rheumatoid arthritis. Pharmacoepidemiol Drug Saf. 2012;21:573. doi: 10.1002/pds.3221. [DOI] [PubMed] [Google Scholar]
- 21.Grammatico-Guillon L., Baron S., Gaborit C., Rusch E., Astagneau P. Quality assessment of hospital discharge database for routine surveillance of hip and knee arthroplasty-related infections. Infect Control Hosp Epidemiol. 2014;35:646. doi: 10.1086/676423. [DOI] [PubMed] [Google Scholar]
- 22.Grammatico-Guillon L., Baron S., Rosset P. Surgical site infection after primary hip and knee arthroplasty: a cohort study using a hospital database. Infect Control Hosp Epidemiol. 2015;36:1198. doi: 10.1017/ice.2015.148. [DOI] [PubMed] [Google Scholar]
- 23.Hanf M., Quantin C., Farrington P. Validation of the French national health insurance information system as a tool in vaccine safety assessment: application to febrile convulsions after pediatric measles/mumps/rubella immunization. Vaccine. 2013;31:5856. doi: 10.1016/j.vaccine.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 24.Moulis G., Lapeyre-Mestre M., Palmaro A. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411. doi: 10.1016/j.revmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Raguideau F., Mezzarobba M., Zureik M. Compliance with pregnancy prevention plan recommendations in 8672 French women of childbearing potential exposed to acitretin. Pharmacoepidemiol Drug Saf. 2015;24(5):526. doi: 10.1002/pds.3763. [DOI] [PubMed] [Google Scholar]
- 26.Bouillon K., Bertrand M., Maura G. Risk of bleeding and arterial thromboembolism in patients with non-valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non-vitamin K-antagonist oral anticoagulant: a retrospective, matched-cohort study. Lancet Haematol. 2015;2(4):e150. doi: 10.1016/S2352-3026(15)00027-7. [DOI] [PubMed] [Google Scholar]
- 27.Maura G., Blotière P.O., Bouillon K. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015;132:1252. doi: 10.1161/CIRCULATIONAHA.115.015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tricotel A., Collin C., Zureik M. Impact of the sharp changes in the use of contraception in 2013 on the risk of pulmonary embolism in France. J Thromb Haemost. 2015;13:1576. doi: 10.1111/jth.13053. [DOI] [PubMed] [Google Scholar]
- 29.Weill A., Dalichampt M., Raguideau F. Low-dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: a cohort study. BMJ. 2016;353:i2002. doi: 10.1136/bmj.i2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raguideau F., Lemaitre M., Dray-Spira R., Zureik M. Association between oral fluoroquinolone use and retinal detachment. JAMA Ophthalmol. 2016;134:415. doi: 10.1001/jamaophthalmol.2015.6205. [DOI] [PubMed] [Google Scholar]
- 31.Bouillon K., Bertrand M., Boudali L. Short-term risk of bleeding during heparin bridging at initiation of vitamin K antagonist therapy in more than 90 000 patients with nonvalvular atrial fibrillation managed in outpatient care. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beziz D., Colas S., Collin C., Dray-Spira R., Zureik M. Association between exposure to benzodiazepines and related drugs and survivorship of total hip replacement in arthritis: a population-based cohort study of 246,940 patients. PloS One. 2016;11:e0155783. doi: 10.1371/journal.pone.0155783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchgesner J., Lemaitre M., Rudnichi A. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: analysis of the French administrative health databases 2009-2014. Aliment Pharmacol Ther. 2017;45:37. doi: 10.1111/apt.13835. [DOI] [PubMed] [Google Scholar]
- 34.Fagot J.P., Blotière P.O., Ricordeau P. Does insulin glargine increase the risk of cancer compared with other basal insulins? A French nationwide cohort study based on national administrative databases. Diabetes Care. 2013;36:294. doi: 10.2337/dc12-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colas S., Allalou A., Poichotte A. Exchangeable femoral neck (dual-modular) THA prostheses have poorer survivorship than other designs: a nationwide cohort of 324,108 patients. Clin Orthop Relat Res. 2017 doi: 10.1007/s11999-017-5260-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozic K.J., Lau E., Ong K. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014;472(2):449. doi: 10.1007/s11999-013-3081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prokopetz J.J., Losina E., Bliss R.L. Risk factors for revision of primary total hip arthroplasty: a systematic review. BMC Musculoskelet Disord. 2012;13(1):251. doi: 10.1186/1471-2474-13-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rey G., Jougla E., Fouillet A., Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997-2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33. doi: 10.1186/1471-2458-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foote J., Panchoo K., Blair P., Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500. doi: 10.1308/003588409X432356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Röttger J., Scheller-Kreinsen D., Busse R. Patient-level hospital costs and length of stay after conventional versus minimally invasive total hip replacement: a propensity-matched analysis. Value Health. 2012;15:999. doi: 10.1016/j.jval.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Stargardt T. Health service costs in Europe: cost and reimbursement of primary hip replacement in nine countries. Health Econ. 2008;17(1 Suppl):S9. doi: 10.1002/hec.1328. [DOI] [PubMed] [Google Scholar]
- 42.Manley M., Ong K., Lau E., Kurtz S. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90(11):2446. doi: 10.2106/JBJS.G.01300. [DOI] [PubMed] [Google Scholar]
- 43.Losina E., Barrett J., Mahomed N.N., Baron J.A., Katz J.N. Early failures of total hip replacement: effect of surgeon volume. Arthritis Rheum. 2004;50(4):1338. doi: 10.1002/art.20148. [DOI] [PubMed] [Google Scholar]
- 44.Khatod M., Barber T., Paxton E., Namba R., Fithian D. An analysis of the risk of hip dislocation with a contemporary total joint registry. Clin Orthop Relat Res. 2006;447:19. doi: 10.1097/01.blo.0000218752.22613.78. [DOI] [PubMed] [Google Scholar]
- 45.Wolford M, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. NCHS Data Brief No. 186, 2015, https://www.cdc.gov/nchs/products/databriefs/db186.htm [accessed 27.01.17]. [PubMed]
- 46.Morshed S., Bozic K.J., Ries M.D., Malchau H., Colford J.M. Comparison of cemented and uncemented fixation in total hip replacement: a meta-analysis. Acta Orthop. 2007;78(3):315. doi: 10.1080/17453670710013861. [DOI] [PubMed] [Google Scholar]
- 47.Parvizi J., Saleh K.J., Ragland P.S., Pour A.E., Mont M.A. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79(3):335. doi: 10.1080/17453670710015229. [DOI] [PubMed] [Google Scholar]
- 48.Agabiti N., Picciotto S., Cesaroni G. The influence of socioeconomic status on utilization and outcomes of elective total hip replacement: a multicity population-based longitudinal study. Int J Qual Health Care. 2007;19(1):37. doi: 10.1093/intqhc/mzl065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.