Abstract
Introduction:
Consensus hasn’t been yet achieved about optimal dose quantity that could prevent post therapy hypothyroidism, thus dosing approach varies among different centers. I131 doses can be fixed or calculated, although treatment outcomes don’t differ significantly according to recent acknowledgments.
Aim:
Determination of the incidence of hypothyroidism after radioiodine treatment (I131) in dependence of hyperthyroidism etiology and quantity of applied doses.
Materials and methods:
The study included 100 patients which have had radioiodine treatment, with a three year post-treatment follow up. The study was conducted at the Nuclear Medicine Department, University Clinical Center of Sarajevo. Data were provided from the patient medical histories. Research is designed as a retrospective, descriptive study. All data were analyzed using Microsoft Excel and the statistical program SPSS 13.0.
Results:
After the three year follow up, incidence of hypothyroidism within patients with Graves’ disease was 89.5%, with diffuse goiter 50%, with toxic adenoma (TA) 26.8%, and with multinodulare goitre (MNG) 57.1%. Hypothyreoidism in patients with diffuse goiter, Graves’ disease and TA was mostly developed after I131 therapy with a dose quantity of 10.1-15 mCi and in MNG patients after RAI therapy with applied doses of 15.1-20 mCi.
Conclusion:
The hypothyroidism incidence rate is the highest among patients with Graves’ diseases and the lowest among the TA patients. It’s mostly developed after dose quantity of 10.1-15mCi and it is rare at dose quantity less than 5mCi. 50% of hypothyroidism were developed among patients with diffuse goiter, Graves’disease.
Keywords: I131 therapy, hyperthyreoidism treatment, incidence of hypotihyeoidism
1. INTRODUCTION
Radioactive iodine I131 (RAI) is used in therapy of hyperthyreoidism since 1940. It is used as one of several different approaches in treating hyperthyreoidism at the beginning of therapy or in relapse of the disease after thyreostatic therapy or surgery, and also in patients with severe adverse effects caused by thyreostatic therapy. In USA, RAI is mostly the first choice for Graves disease therapy, while in Europe it is usually used as definitive treatment (1).
Trapped in thyreocytes I131 leads to destruction of these cells, which is the main mechanism of its effects. There are different techniques for calculating the proper dose of I131, mostly based on its uptake test and ultrasound determined gland volume. The other approach is to apply fix, empirical doses. Usage of empirical doses is widely accepted as there is no valid data that could prove superiority over the calculated doses in prevention of hypothyreosis (2).Therapeutic approach of fixed dose usage is also much more economical.
According to ATA and ETA guidelines it is necessary to avoid iodine intake at least 7 days before the treatment (3). In patients treated with radioactive iodine or surgery, it is mandatory to check thyroid hormones periodically due to high incidence of hypothyreoidism in these patients.
In Lewis’s study, outcome of fixed I131 dose of 550 MBq was 74,4 % of hypothyroid patients up to one year after the treatment. For the same period of time, the incidence of hypothyreosis after the treatmnet is higher in patients with Graves disease (78%) (5).
Radioactive I131 treatment is also used in treating of multinodular goitre (MNG) and toxic adenoma (TA). Development of hypothyreoidism in these patients after I131 therapy is common but more rare than in patients with Graves disease. I131 dose used in therapy is usually higher for MNG and TA than those for Graves disease although optimal doses differ among centers (6).
Szumowski and collegues conducted a study revealing outcomes after I131 treatment of patients with MNG and TA: 3% of patients with TA and 4% of patients with MNG developed hypothyreosis in the first posttreatment year (7).
Use of radioactive I131 therapy has significantly decreased the need for operative treatment, and the method is easier, painless and minimally unpleasent for patients. Depending on hyperthyreosis etiology and I131 activity used, incidence of hypothyreoidism can vary.
2. AIM
The aim of our study is to evaluate primary causes of hyperthyreoidism, to calculate avarage doses of I131 used in therapy and to calculate the incidence of hypothyreoidism in follow up after I131 therapy depending on hyperthyreoidism etiology. We are also going to investigate the I131 dose activity calculation approachand impact on hypothyreoidis, incidence.
We assume that hypothyreoidism incidence after I131 treatment depends on hyperthyreoidism etiology and on the I131 dose level.
3. MATHERIALS AND METHODS
There were 100 patients included in this study. The study was conducted in the Clinical Center of the University in Sarajevo, Nuclear Medicine Department. Patients were treated with I131 therapy and regulary followed up for the period of three years.
Patients were divided in 5 groups according to hyperthyreodism etiology (TA, MNG, Graves disease, diffuse hyperthyroidism without elevated antibodies and hyperthyreoidism due to postoperative relapse). Hormonal status was checked after one, six, twelwe, twenty four and thirty six months post therapy.
For statistical needs, patients were divided into two groups depending on the kind of the I131 dose which was applied, calculated or empirical.
The study is designed as a retrospective descriptive study, based on randomised samples. All data used is extracted from the medical archive of the Nuclear medicine department, including anamnestic data, physical and laboratory findings.
Patients were classified as euthyroid, hypothyroid, subclinicaly hypothyroid or hyperthyroid based on laboratory findings.
Normal ranges were as follows: TSH 0,3-4,2 mU/l; FT4 12,0-22,0 pmol/l; FT3 3,1-6,8 pmol/l; antiTg<115IU/ml; antiTPO<34IU/ml.
Diagnosis of thyroid disease was established by clinical, laboratory and a scintigraphic exam. Thyroid scintigraphy was performed on a one head camera Mediso, and a two headed gamma camera Siemens in the Nuclear medicine clinic.
In selected patients, I 131 dose activity was measured using test capsules, and using the following formula:
Empirical doses were used based on hyperthyreoidism etiology and thyroid volume. Pre-treatment protocol included obtainance of informed consent, negative pregnancy test, ultrasound measured thyroid or nodule volume, hormonal status and thyroid scintigraphy. All patients stopped taking tireostatic therapy 5-7 days before therapy.
Patients with Graves disease received cosrticosteroid therapy two days prior to I131 therapy in a dose of 40mg/24h. All patients received a informative leaflet regarding the period after I131 therapy. All patients were on a low dose iodine diet ten days prior to therapy and two days after.
Statistical analysis
The normality of distribution was assessed by the Kolmogorov-Smirnov test, or Shapiro Wilkinson test, based on sample size. Chi- square test was used to test frequencies of occurence. Normally distributed data was presented as mean value () and standard error of mean value (SEM) and skewed variables as median and interquartile range. The Student T-test or ANOVA are used to compare differences between the groups, as appropriate.
Description and statistical analyses were performed by Microsoft office Excel 2003 and SPSS version 13.0. for Windows (SPSS Inc., Chicago, IL, USA).
Probability value of <0.05 was considered statistically significant.
4. RESULTS
Etiology of hyperthyreoidism in patients included in the study
Prior to I131 therapy 14 patients had diffuse hyperthyroidism, 19 (19%) had Graves disease, 56 (56%) had toxic adenoma (TA), 7 (7%) multinodular goitre and 4 (4%) had postoperative relapse of hyperthyreoidism (Figure 1).
Figure 1.
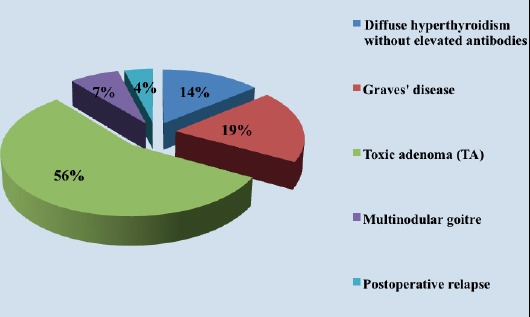
Percentage of patient distribution based on hyperthyreoidism etiology
Radioactive I131 therapy
Avarage dose of I131 used for therapy in our patients was 14.15±0.43 mCi. Minimal dose activity was 5 mCi, and maximal dose used was 27 mCi.
Calculated I131 doses were used in 68 patients, and empirical doses were used in 32 patients (Figure 2).
Figure 2.
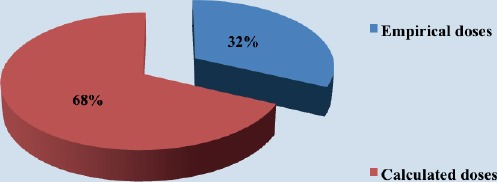
Percentage of I131empirical and calculated doses used in therapy
Avarage dose of I131 empirical activity used in the study was 13.91±0.7 mCi. Minimal empirical dose used was 5 mCi, and maximal empirical dose was 20 mCi.
Avarage I131 calculated dose activity was 14.26±0.55mCi. Minimal calculated dose used in the study was 4.5 mCi, and maximal dose was 27 mCi.
Difference in I131 empirical and calculated dose activity used in the study was not statisticaly significant (p=0.705; p>0.05) (Figure 3).
Figure 3.
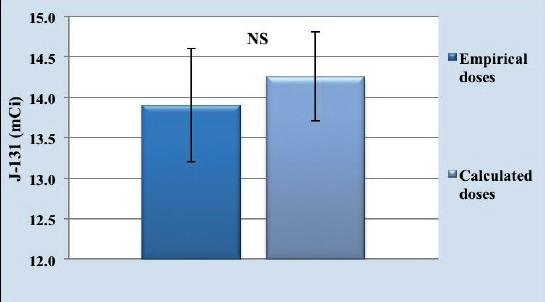
Avarage activity of empirical and calculated doses used in therapy
Mean value±mean value standard error (±SEM) of empirical and calculated I131 doses is not significant
Established level of applied I131 doses in patients with diffuse goitre was statistically significantly lower comparing to patients diagnosed with TA (p*=0.003; p<0.05), as well as comparing to patients with multinodular goitre (p**=0.013;p<0.05) and patients with Graves disease (p***=0.028; p<0.05) (Figure 4).
Figure 4.
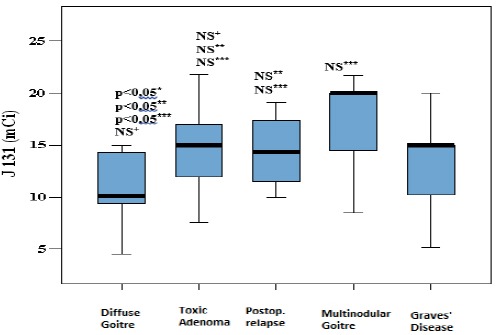
Applied I131 dose in dependance on primary disorder
Median, 25th and 75th percentile of I 131 doses are presented, regarding primary cause of hyperthyoridism. Whiskers present 1st and 3rd Quartile, and Box stands for Interquartile Range and Median. P value less than 0.05 is considered significant is non significant; *-in relation to TA; **- in relation to MNG; ***- in relation to Graves’ disease; +- in relation to postoperative relapse.
Hypothyreoidism depending on type of applied dose (calculated or empirical)
By analysing patients with different etiology of hyperthyreosis, which have been hypothyreoid at least once depending on type of applied I131 dose, we concluded that among 54-100% hypothyroid patients, usage of calcualted doses was higher (34-62.96%) comparing to usage of empirical doses(20-37.03%). Established difference in hypothyreosis incidence depending on the type of the applied dose was marginaly statistically significant (χ2=3.63; p=0.057) (Figure 5, 6).
Figure 5.
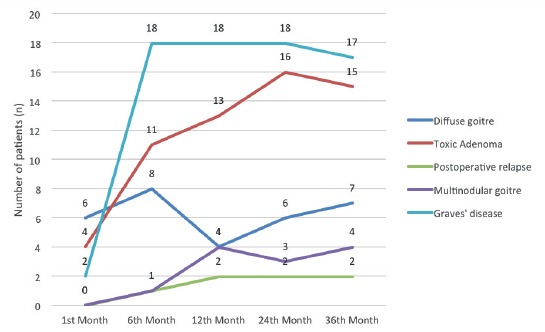
Hypothyreoidism incidence in three years follow up in dependance of primary diagnosis
Figure 6.
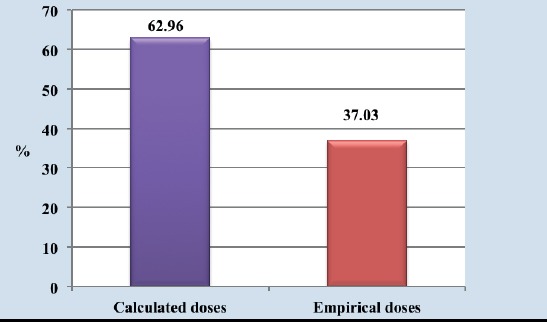
Incidence of hypothyreoidism depending on applied method of dose calculation
5. DISSCUSSION
Our study was conducted in order to correlate etiology of hyperthyreoidism and the type of applied doses on one side and incidence of hypothyreosis on the other side.
The results showed that the most frequent primary cause of hyperthyreoidism in our patients was solitary toxic adenoma (TA) with 56%, following Graves disease with 19 % cases and diffuse goitre with 14%. According to the other authors, the most frequent cause of hyperthyroidism was Graves’ disease, but these studies included higher number of patients compared to our sample which could explain the differences in percentages of hyperthyroidism etiology (8).
I131 dose was calculated individually, according to iodine uptake in 68 patients, while 32 patients have received a empirical fixed I131 dose. Analysing these results, we found that the difference in hypothyreoidism incidence in dependance on type of applied dose was borderly statistically significant (x2=3,63; p=0,057).
According to the literature, there are no available data that calculated doses are better than empirical in the sense of decrease of hypothyroidism development, which correlates with our results. This is why many centers, including ours, use empirical doses due to practical and economical reasons.
According to results of metaanalysis published in 2014. there were no significant differences in hypothyroidism development in patients with nodular goitre receiving a calculated and empirical dose (0.3% higher incidence of hypothyroidism when calculated dose applied (9).
Similar results were found by Canadian authors in Graves’ disease treated with I131. They concluded that type of applied I131 dose had no significant impact on hypothyroidism incidence (10).
Analysing hormonal status of patients included in this study during three years post-treatment follow up, in dependance of hyperthyreoidism etiology, it is concluded that the highest incidence of hypothyreoidism was recorded in patients with Graves disease reaching 94,7% in 6th month post-treatment. Our results correlates with a study conducted in 2013 by John Enyi Ejeha and collegues which showed also the highest incidence of hypothyreoidism in the 6th month post-treatmen in patients with Graves disease (11). Lewis and collegues presented their results showing hypothyreoidism incidence of 78% in patients one year after RAI therapy (12).
In patients with toxic adenoma as primary cause of hyperthyroidism, we found the majority of patients euthyroid after RAI treatment. Similar results were published in the study by Szumowski and collegues, where the highest incidence of euthyroidism (94%) one year after I131 therapy, was found in patients with TA and this incidence is significantly lower comparing to Graves diseasse and diffuse goitre (13).
6. CONCLUSION
Etiology and quantity of applied I131 dose influence hypothyroidism incidence increase, and the highest incidence of hypothyroidism after RAI therapy was in patients with Graves disease treated with doses of 10-15 mCi of I131.
It is necessary to find best approach for decrease of hypothyroidism incidence after the I131 therapy and its eventual usage in clinical management of patients treated with RAI. Results are suggesting there should be a analysis of optimal doses correction, in a way of decreasing hypothyreoidism development without decreasing I131 dose efficcacy.
Footnotes
• Conflict of Interest: none declared.
• Authors contribution: Nermina Beslic and Sabrina Licina participate in study design and acquisition, analysis and interpretation of data. Authors equally participate in drafting the article and give final approval for the version which is submitted.
REFERENCES
- 1.Prakash A, Shamasunder A. Current and emerging treatment options for Graves'hyperthyroidism. Ther Risk Clin Manag. 2010;6:29–40. doi: 10.2147/tcrm.s5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré J. The Incidence and Prevalence of Thyroid Dysfunction in Europe: A Meta-Analysis. The Journal of Clinical Endocrinology & Metabolism. 2014;99(3):923–31. doi: 10.1210/jc.2013-2409. [DOI] [PubMed] [Google Scholar]
- 3.European Th yroid Association. World Thyroid Day. 2013. Available: http://www.eurothyroid.com/admin/dc_media/WTD_Statement_May_2013 . retreived.
- 4.Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: Management Guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:457–520. doi: 10.4158/ep.17.3.456. [DOI] [PubMed] [Google Scholar]
- 5.Lewis A, Atkinson B, Bell P, Courtney H, McCance D, Mullan K, Hunter S. Outcome of 131I therapy in hyperthyroidism using a 550MBq fixed dose regimen.Ulster Med J. May. 2013;82(2):85–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: Management Guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:457–520. doi: 10.4158/ep.17.3.456. [DOI] [PubMed] [Google Scholar]
- 7.Szumowski P, Rogowski F, Abdelrazek S, Kociura-Sawicka A, Sokolik-Ostasz A. Iodine isotope ¹³¹I therapy for toxic nodular goitre: treatment efficacy parameters. Nucl Med Rev Cent East Eur. 2012 Apr 24;15(1):7–13. doi: 10.5603/nmr-18724. [DOI] [PubMed] [Google Scholar]
- 8.Enyi Ejeh M.J, Omotayo Ogunjobi K, Enyi Ejeh J, Solomon Adedapo K, F Eniojukan J. Effectiveness of Fixed Dose Radioactive Iodine (RAI) for the Treatment of Hyperthyroidism: Experience of a Teaching Hospital in South West Nigeria. Mol Imaging Radionucl Ther. 2013 Aug;22(2):36–41. doi: 10.4274/Mirt.08370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rokni H, Sadeghi R, Moossavi Z, Treglia G, Zakavi SR. Efficacy of Different Protocols of Radioiodine Therapy for Treatment of Toxic Nodular Goiter: Systematic Review and Meta-Analysis of the Literature. Int J Endocrinol Metab. 2014 Apr 1;12(2):e14424. doi: 10.5812/ijem.14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leslie W, Ward L, Salamon E, Ludwig S, Rowe R, Cowden E. A Randomized Comparison of Radioiodine Doses in Graves'Hyperthyroidism. The Journal of Clinical Endocrinology & Metabolism. 2003;88(3):978–83. doi: 10.1210/jc.2002-020805. [DOI] [PubMed] [Google Scholar]
- 11.Enyi Ejeh MJ, Omotayo Ogunjobi K, Enyi Ejeh J, Solomon Adedapo KF, Eniojukan J. Effectiveness of Fixed Dose Radioactive Iodine (RAI) for the Treatment of Hyperthyroidism: Experience of a Teaching Hospital in South West Nigeria. Mol Imaging Radionucl Ther. 2013 Aug;22(2):36–41. doi: 10.4274/Mirt.08370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis A, Atkinson B, Bell P, Courtney H, McCance D, Mullan K, Hunter S. Outcome of 131I therapy in hyperthyroidism using a 550MBq fixed dose regimen. Ulster Med J. 2013 May;82(2):85–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Szumowski P, Rogowski F, Abdelrazek S, Kociura-Sawicka A, Sokolik-Ostasz A. Iodine isotope ¹³¹I therapy for toxic nodular goitre: treatment efficacy parameters. Nucl Med Rev Cent East Eur. 2012 Apr 24;15(1):7–13. doi: 10.5603/nmr-18724. [DOI] [PubMed] [Google Scholar]


