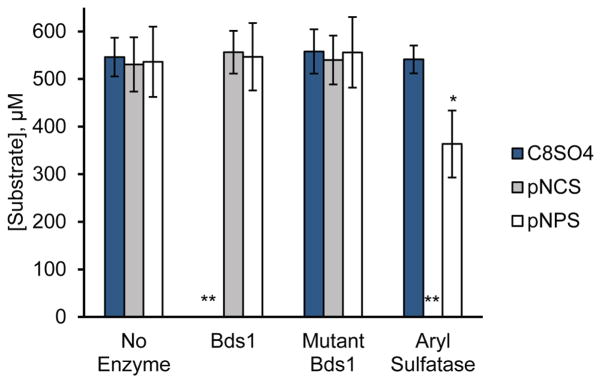
Fig. 3.
Bds1 hydrolyzes primary alkyl but not aryl sulfates. The substrate concentration remaining after ~50 h reaction of 500 μM substrate with 1.0 μM enzyme is shown for the substrates 1-octyl sulfate (C8SO4), p-nitrocatechol sulfate (pNCS), and p-nitrophenyl sulfate (pNPS) in the absence of enzyme (No Enzyme) or in the presence of Bds1, the catalytic mutant Bds1(D166N, H167A) (Mutant Bds1), or 74 mg L−1 aryl sulfatase from Patella vulgata (Aryl Sulfatase) in 10 mM tris-HCl, pH 7.4. Substrate concentrations following reaction were measured by LC-MS. Bars and error bars indicate the average and standard deviation of three independent biological replicates, each analyzed with three technical replicates. *p<0.0001 compared to the same substrate in the presence of Bds1 or the Bds1 catalytic mutant or in the absence of enzyme. **Substrate levels at the end of these reactions were below the limit of detection, i.e., less than 1 μM substrate.