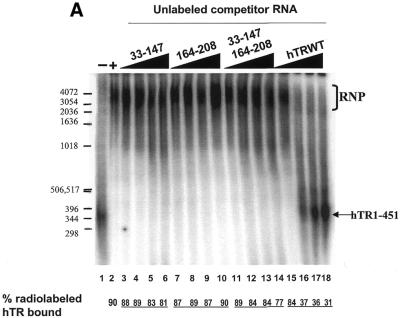
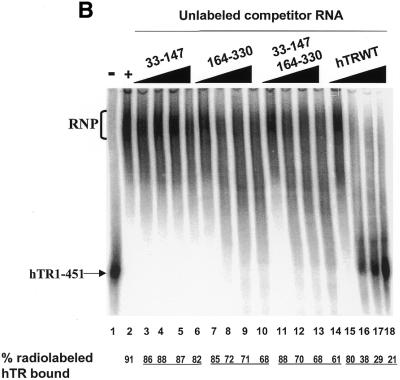
Figure 4.
hTR33–147 and hTR164–330 do not cooperate to inhibit the formation of the hTR–protein telomerase complex as efficiently as wild-type hTR. (A) Effect of hTR33–147 and hTR164–208 on hTR–protein telomerase complex formation in vitro. 32P-labeled hTR (0.25 pmol) was incubated in the absence (lane 1) or presence (lanes 2–18) of 3.4 µg partially purified telomerase extracts from 293 cells. Standard binding reactions were performed in the absence (lane 2) or presence (lanes 3–18) of increasing concentrations (0.05, 0.4, 0.8 and 1.6 µM) of unlabeled hTR33–147 (lanes 3–6), hTR164–208 (lanes 7–10), hTR33–147 with hTR164–208 (lanes 11–14), and wild-type hTR (lanes 15–18). The arrow points to free radiolabeled wild-type hTR, and the bracket indicates the RNP complex. The percentage (%) of radiolabeled complex-bound hTR is indicated at the bottom for each lane. DNA molecular weight standards are indicated on the left (in bp). (B) Effect of hTR33–147 and hTR164–330 on hTR–protein telomerase complex formation in vitro. 32P-labeled hTR (0.25 pmol) was incubated in the absence (lane 1) or presence (lanes 2–18) of 3.4 µg partially purified telomerase extracts from 293 cells. Standard binding reactions were performed in the absence (lane 2) or presence (lanes 3–18) of increasing concentrations (0.05, 0.4, 0.8 and 1.6 µM) of unlabeled hTR33–147 (lanes 3–6), hTR164–330 (lanes 7–10), hTR33–147 with hTR164–330 (lanes 11–14), and wild-type hTR (lanes 15–18). The arrow points to free radiolabeled wild-type hTR and the bracket indicates the RNP complex. The percentage of radiolabeled complex-bound hTR is indicated at the bottom for each lane.