Abstract
Visual impairment can complicate cerebral venous thrombosis (CVT). Here, we describe the various pathophysiological mechanisms and treatments available. A retrospective chart review of all patients treated for CVT in a large quaternary teaching hospital was done, and cases with visual impairment due to CVT were identified. The various mechanisms causing visual impairment in CVT were (1) raised intracranial pressure (ICP) caused by venous thrombosis without venous infarcts resulting in a benign intracranial hypertension-like presentation of CVT, (2) venous infarcts involving the occipital cortex, (3) raised ICP following the development of a secondary dural arteriovenous (AV) fistula, and (4) arterial occipital infarcts due to posterior cerebral artery compression secondary to herniation in large venous infarcts. Apart from using systemic anticoagulants to attempt recanalization and drugs with carbonic anhydrase inhibitor activity to reduce the ICPs, treatment modalities employed to save vision were (1) recanalization by local thrombolysis, stenting, or mechanical devices; (2) cerebrospinal fluid diversion procedures such as theco-periotoneal shunting; (3) optic nerve sheath fenestration; and (4) specific treatment for conditions such as dural AV fistula occurring as a late complication. CVT can cause visual impairment through different pathophysiological mechanisms. Depending on the mechanism, treatment strategies need to be tailored. Furthermore, very close monitoring is needed both in the acute and in the follow-up period, as new pathophysiological mechanisms can arise, compromising the vision. This may require a different treatment approach. Literature on this aspect of CVT is lacking.
Keywords: Cerebral venous thrombosis, dural arteriovenous fistula, occipital venous infarcts, optic nerve sheath fenestration
INTRODUCTION
Cerebral venous thrombosis (CVT) usually affects the younger population.[1] Earlier it was a condition with high mortality and morbidity.[2] Now, with early diagnosis using better imaging modalities and better understanding of the pathophysiology, the outcome has improved. However, 13% of the patients can still either die or become dependent.[1]
One of the dreaded complications of CVT is visual loss. There have been many studies looking at various aspects of CVT such as mortality, risk factors, recurrence, and treatment; however, literature on visual complication and its treatment is lacking.
In the VENOPORT study, Ferro et al.[3] reported visual impairment in 13% patients, with severe visual loss occurring in 1.4%.
In a retrospective study from India, Nithyanandam and Bhargava[4] noted that 10% of CVT patients had transient visual obscuration at the time of presentation.
There are many mechanisms of visual impairment in CVT: (1) optic nerve dysfunction secondary to raised intracranial pressure (ICP) – benign intracranial hypertension such as presentation of CVT;[5] (2) venous infarcts in the occipital cortex; (3) raised ICP on the optic nerve following the development of a secondary dural AV fistula following the CVT; and (4) bilateral or unilateral arterial occipital infarcts due to posterior cerebral artery (PCA) compression in survivors of uncal herniation in large venous infarcts. Furthermore, rarely, an underlying disease such as antiphospholipid antibody syndrome can affect the vision and also cause the CVT.
For each of these mechanisms, a different treatment strategy may have to be employed. Literature on this aspect of CVT is lacking. A good understanding of these various pathophysiological mechanisms is essential for planning the best treatment strategies for each of them till future clinical trials and studies give us more clarity.
Here, we have illustrated these various mechanisms and how they can be best managed.
BENIGN INTRACRANIAL HYPERTENSION-LIKE PRESENTATION OF CEREBRAL VENOUS THROMBOSIS
Optic nerve dysfunction secondary to raised ICP can mimic benign intracranial hypertension.[5] Nearly 37% to 47% of patients with CVT can present with features indistinguishable from typical idiopathic intracranial hypertension (IIH).[6] In patients with presumed IIH, 10% had CVT when magnetic resonance imaging (MRI)/magnetic resonance venography (MRV) was performed.[7]
The pathophysiology here is subacute to chronic occlusion of the dural venous sinuses [Figure 1]. When there is an occlusion in the cerebral dural sinuses, there is congestion in all the cortical veins which drain into them [Figure 1a]; this extra venous blood will cause an increase in the ICP. The initial compensatory mechanism for this rise in the ICP is to decrease the cerebrospinal fluid (CSF) volume by increasing the CSF drainage.[8] Since the drainage for the CSF is through the arachnoid granulations located in the superior sagittal sinus (SSS) and transverse sinus, this buffer mechanism fails if these sinuses are thrombosed [Figure 1b], and the extra CSF will also add to the raised ICP. When the occlusion of the dural venous sinuses occurs as a chronic process over a period of time, multiple draining veins can get occluded with the ICP steadily increasing and causing symptoms. Furthermore, over a period of time, multiple collateral channels will open up increasing the volume of venous blood and further raising the ICP [Figure 1c].
Figure 1.
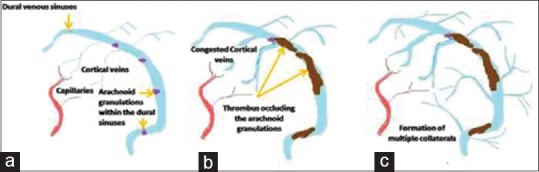
The pathophysiology of benign intracranial hypertension-like presentation of cerebral venous thrombosis (a) arachnoid granulations mainly located in the superior sagittal and the transverse sinuses (b) thrombus occluding the arachnoid granulations and decreasing the cerebrospinal fluid reabsorption (c) formation of multiple collateral channels
This raise in the ICP will get reflected on the optic nerve and cause optic nerve dysfunction.
Experiments by Hayreh[9] have shown that the rise of CSF pressure in the optic nerve sheath causes axoplasmic flow stasis in the nerve fiber, resulting in swelling of the nerve fiber and the optic disc. This in turn causes venous stasis and leakage and accumulation of extracellular fluid.[10] Early symptoms can be headache and features of raised ICP. The patient can also have episodes of monocular or binocular transient visual blurring,[11] (visual obscuration), which usually lasts for <30 s. Pulsatile tinnitus[12] (pulse synchronous tinnitus) can also occur. This can progress to visual field deficits and worsening of the visual acuity and eventually blindness in one or both the eyes.
The main target of therapy in this subgroup of patients with CVT should be to reduce the effect of the raised ICP on the optic nerve.
Close subjective and objective monitoring should be done and the treatment strategy modified if needed.
In the acute phase, resolution of papilledema and periodic ultrasonic monitoring of the retrobulbar optic nerve sheath diameter (ONSD) can help in guiding therapy.[13] On follow–up, pupillary light reflex and field charting should be done.
Apart from the standard treatment for attempting recanalizing of the thrombosed sinuses and veins using intravenous unfractionated heparin (UFH) or subcutaneous low-molecular-weight heparin (LMWH),[14,15] the raised ICP has to be reduced with the use of drugs with carbonic anhydrase inhibitor activity such as acetazolamide or topiramate to decrease CSF production. The treatment may require one or a combination of the following treatment methods.
Recanalization by local thrombolysis, stenting, or mechanical devices
Thrombolytic agents such as urokinase, streptokinase, and tissue plasminogen activator can be used to achieve recanalization. However, in CVT unlike in arterial strokes, the thrombus load is very high; hence, larger dose of these agents will be required to bring about an effective thrombolysis with a higher risk of bleeding complications.[16] Directly delivering these agents into the blocked dural sinuses (direct catheter thrombolysis) may achieve thrombolysis with lesser bleeding complications. Furthermore, recanalization of the occluded sinuses can be achieved by stenting or using mechanical thrombectomy devices which are used in the treatment of acute ischemic stroke.[17]
There is an ongoing randomized trial comparing anticoagulation with local thrombolysis in patients with severe acute CVT.[18]
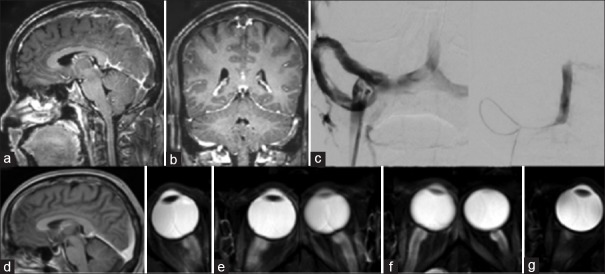
Case 1 [Figure 2]
Figure 2.
(a) T1 gado sagittal and (b) coronal sections showing thrombosis involving the superior sagittal, bilateral transverse, and sigmoid sinuses (c) angioplasty and stenting of right transverse sinus. (d) Postprocedure T1 gado sagittal magnetic resonance imaging showing good recanalization (e) posterior scleral surface flattening at admission (f) increase in the flattening with inward bowing associated with clinical deterioration in vision (g) improvement with normal appearing posterior sclera surface 2 months postprocedure
A 40–year-old male presented with 1-week history of severe holocranial headache. Two days before seeking medical aid, the headache had increased in severity with intermittent vomiting, binocular diplopia with horizontal separation of images. He also had intermittent visual obscurations, especially on bending the head forwards. He had no comorbidities. The pupils were bilaterally reactive to light and accommodation. The visual acuity was normal, and the fundus examination showed bilateral papilledema with hemorrhages. MRI brain with contrast [Figure 2a and b] showed extensive acute to early subacute thrombus filling the SSS, transverse sinus, and sigmoid sinus bilaterally. There were features of raised ICP as shown by posterior scleral surface flattening [Figure 2e].
He was started on therapeutic anticoagulation with LMWH, acetazolamide, and antiedema measures. However, he had a persistent headache with diplopia. The initial transient visual obscuration increased in frequency and he developed blurring of vision. He was being monitored with daily ONSD measurements which was showing a marginal increase from 6.3 to 7.5 mm. Repeat imaging showed [Figure 2f] persistent perioptic flare with inward bowing of the posterior scleral surfaces and partially empty sella – indicating worsening of the raised ICPs. Since there was subjective and objective worsening, he was taken up for stenting of the occluded sinus.
A digital subtraction angiography showed significant stenosis and occlusion of the right transverse sinus. Stenting of the right transverse sinus was done with 6 mm × 18 mm and 5 mm × 18 mm stents. Following this, the pressure in the SSS reduced from 67 mmHg to 18 mmHg. Over the next 48 h, the patient had good clinical improvement with resolution of lateral rectus palsy and diplopia and improvement in vision as well. A repeat imaging done after 2 months shows partial recanalization [Figure 2d] of the sinuses and normal appearing posterior sclera [Figure 2g].
Cerebrospinal fluid diversion procedures to decrease the intracranial pressure
Occasionally, serial lumbar puncture to removal CSF can be attempted carefully if there is no risk of herniation.[19] In refractory cases, a lumboperitoneal shunt can be done.[20]
However, overdrainage of CSF following the lumboperitoneal shunt is a known complication.[21]
Among six patients who underwent TPS for a BIH-like presentation of CVT, Kulkarni et al.[22] reported symptomatic excessive CSF drainage as a complication in two patients.
Furthermore, if the overdrainage results in a severe intracranial hypotension, it can potentially cause a subdural hematoma or hygroma due to rupture or leakage of blood from the bridging veins.[23]
The possibility of this complication is increased in the setting of a chronic CVT (case 2 and case 3) where there can be multiple collateral formation with the emissary veins which connect the extracranial venous system with the intracranial venous sinuses becoming prominent and turgid. In such a setting when there is a sudden downward sagging of the brain following an intracranial hypotenion due to overdrainage, there is the potential danger of these bridging veins rupturing resulting in subdural hemorrhage; it is always prudent to use a lumboperitoneal shunt with an adjustable valve to prevent overdrainage and its subsequent complications.
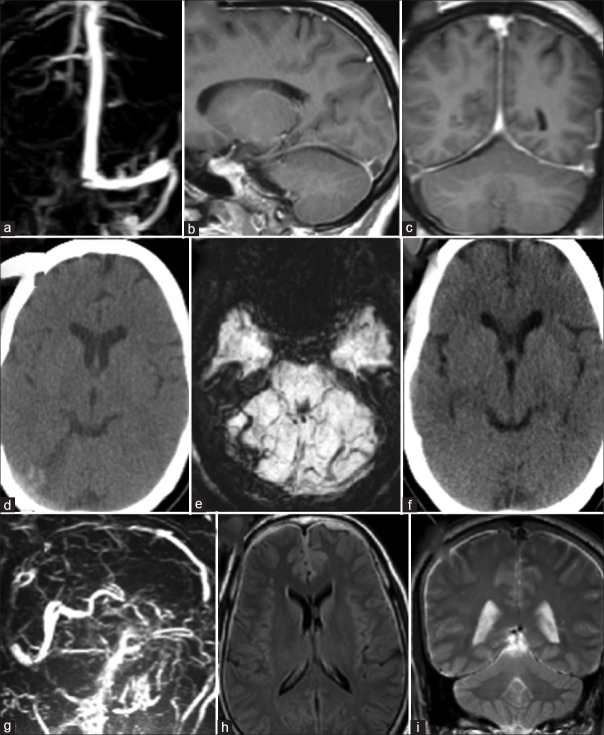
Case 2 [Figure 3a–f]
Figure 3.
(a) Gado-enhanced magnetic resonance venography, (b) T1 gado sagittal and (c) T1 gado coronal images showing thrombosis involving right transverse and sigmoid sinuses (d) plain computerized tomography scan and (e) susceptibility weighted imaging magnetic resonance imaging showing small subdural collection along the right cerebellar hemisphere and tentorium (f) plain computerized tomography scans showing good resolution of the subdural collection (g) magnetic resonance venography showing chronic venous thrombosis with extensive collateral formation postlumbar peritoneal shunt (h) fluid-attenuated inversion recovery and (i) T2 weighted images showing thin subdural hematoma
A 33-year-old female presented with complaints of recurrent episodes of blurring of vision, each lasting for a few seconds, for 2 weeks’ duration. These episodes were mostly in the morning time while straining for defecation. There was a history of moderate-to-severe holocranial headache of 10 days duration, associated with nonprojectile vomiting (4–5 episodes per day). She also had binocular diplopia for 2 days. Pupils were bilaterally reactive to light and accommodation. There were bilateral papilledema and fields showed an inferior nasal quadrantanopia with left side showing an enlarged blind spot. The visual acuity was right - 6/12 and left side - 6/6 and she had bilateral 6th nerve paresis. MRI brain showed features consistent with subacute cortical venous thrombosis involving right transverse and sigmoid sinuses [Figure 3a–c]. CSF opening pressure was 55 cm of water. Patient's condition did not improve with medical treatment with anticoagulants and antiedema measures, and after 3 weeks, a theco-peritoneal shunt was done. The patient developed severe headache after theco-peritoneal shunt. Since the headache persisted even after 10 days, a plain computerized tomography (CT) scan of the head was done which showed a subdural collection along the right cerebellar tentorium [Figure 3d and e]. Her anticoagulation was temporarily withdrawn. A repeat CT done after 3 weeks showed complete resolution of the subdural [Figure 3f]. Anticoagulant was restarted. Her symptoms gradually improved over the next 6 months.
Case 3 [Figure 3g–i]
A 53-year-male presented with a 4-year history of holocranial headache and progressive decrease in the visual acuity since 11 months. He had bilateral papilledema. His initial MRI was normal. The CSF opening pressure was 44 cm of water. He was treated as a case of benign intracranial hypertension and had improved with acetazolamide. After a year, he had a recurrence of symptoms. He was evaluated and noted to have papilledema (persisting) and also early secondary optic atrophy bilaterally. A repeat imaging showed features of CVT with extensive collateral formation [Figure 3g]. The CSF opening pressure was 40 cm of water. He underwent the coperitoneal shunt. On the 3rd day following the shunt, the patient complained of severe headache. Imaging was done which showed features of a subdural hemorrhage [Figure 3h and i]; the shunt was ligated and the patient improved with symptomatic treatment.
Optic nerve sheath fenestration
Direct decompression of the optic nerve can be attempted in BIH-like presentation of CVT since it is the pressure in the optic nerve sheath which causes the main morbidity.
Creating a small window or making a series of openings on the optic nerve sheath behind the globe releases the pressure on the optic nerve. Dural fistula which is created allows egress of CSF from the operative site. Furthermore, doing optic nerve sheath fenestration (ONSF) in one eye can also potentially decompress the other eye.
ONSF has been used in the treatment of BIH and found to be safe and effective;[24,25] this procedure has also been attempted to treat the raised ICPs in CVT.[26,27]
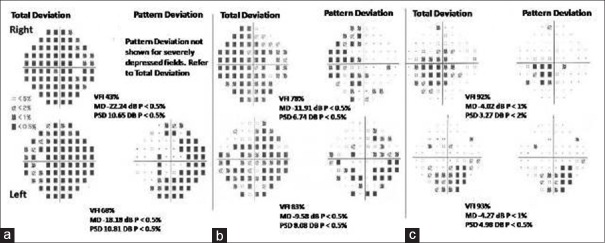
Case 4 [Figure 4]
Figure 4.
(a) The fields showed a generalized depression R > L (b) fields done after 1 month postoptic nerve sheath diameter in the R eye: Showing good improvement on the R side as shown by fewer depressed spots. In the L eye, there is improvement but not as marked as in the R eye. (c) Fields done after 3 months following subsequent optic nerve sheath diameter in the L eye showing improvement in fields in both eyes with a few depressed spots inferiorly
A 31-year-old male presented with a 6-month history of holocranial headache. The headache had increased in severity over the past 6 weeks with transient visual blurring for the same period and a decrease in his visual acuity since 1 week. At the time of his presentation, the vision was 6/12 on both sides. Fundus showed bilateral papilledema with hemorrhages.
The MRI/MRV showed thrombosis of the SSS, the left transverse sinus, and sigmoid sinus without any venous infarcts. He was taken up for a mechanical thrombus maceration and chemical thrombolysis with urokinase. Partial flow in sinus was seen at the end of the procedure. Standard treatment with UFH and acetazolamide was started. The headache improved, but the vision did not improve. After 5 days, the vision dropped to 6/18 and became achromatic in the right and 6/9 in the left eye. The fields showed a generalized depression R > L [Figure 4a]. An ONSD was done on the right side. There was no complication in the immediate postoperative period. After 1 month, the vision improved to 6/6 in both eyes. The color vision had returned with improvement in the papilledema and fields [Figure 4b]. On follow-up after a month, the left eye was not showing significant improvement in fields (as compared to the right) with left disc edema persisting and with the acuity, color vision remaining status quo; hence, ONSD was done on the left side also. On 3-month follow-up, vision had improved to 6/6 in both eyes and vision was trichromatic with a resolution of disc edema in both eyes and field showing improvement with few depressed spots persisting inferiorly in both eyes [Figure 4c].
Visual impairment due to secondary dural arteriovenous fistula
Dural arteriovenous (AV) fistulas are fistulas connecting the branches of dural arteries to dural veins or a venous sinus. Intracranial dural AV fistulas can mimic isolated intracranial hypertension.[28] The mechanism proposed is an increase in the dural sinus pressure, resulting in a secondary diminution of the CSF absorption.[29]
Development of AV fistula can be a late complication of CVT;[30] there have been various theories proposed for this occurrence of AV fistula as a late rare complication of CVT. An increase in sinus and venous pressure can open up channels between the meningeal arterial networks and the dural venous sinuses. Furthermore, the obstruction to venous outflow may reduce cerebral perfusion inducing aberrant angiogenesis resulting in an AV shunt.
This potentially complication should be suspected in CVT patients who develop raised ICP symptoms during their follow-up phase.
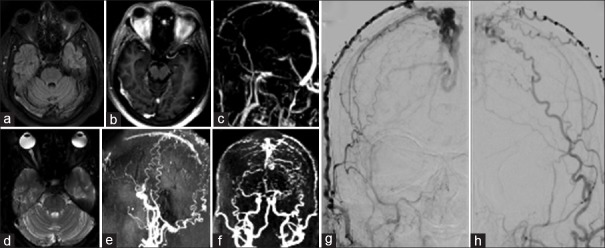
Case 5 [Figure 5]
Figure 5.
(a) Fluid-attenuated inversion recovery (b) gado (c) gado magnetic resonance venography images showing filling defects in mid-superior sagittal, R transverse, and sigmoid sinuses. Magnetic resonance imaging done after 16 months (d) T2, (e and f) magnetic resonance venography/magnetic resonance angiography showing nonvisualization of sinuses with markedly tortuous collaterals suggesting a dural arteriovenous fistula. (g and h) Cerebral angiogram confirming dural arteriovenous fistula in superior sagittal sinus with feeders from bilateral superficial temporal, occipital, middle meningeal artery, and right ophthalmic arteries
A 47-year-old male presented with chronic headache and progressive vision loss of 6-month duration. He was a known case of type 2 diabetes mellitus and systemic hypertension. He was a chronic alcoholic. On examination, vision was 6/9 in the right eye and 6/12 in the left eye and there was bilateral papilledema. MRI brain with contrast showed features of chronic CVT with raised intracranial tension [Figure 5a–c].
He was started on anticoagulation and acetazolamide. There was a rapid worsening of vision and he underwent left optic nerve fenestration. He was continued on acetazolamide and oral anticoagulants. There was gradual improvement in the headache and vision following the procedure. However, after a period of 8 months, his headache recurred, and he also complained of mild worsening of his vision. A repeat MRI was done which showed nonvisualization of the posterior part of the SSS, right transverse sinus, and sigmoid sinus with markedly tortuous collaterals suggesting the possibility of a dural AV fistula [Figure 5d–f].
Cerebral angiogram showed complete obliteration of the SSS, inferior sagittal sinus, right transverse sinus with residual filling in left transverse sinus, and sigmoid sinus with delayed venous drainage through dilated and tortuous cerebral veins bilaterally with dural AV fistula in SSS posteriorly with feeder from bilateral superficial temporal, occipital, middle meningeal artery, and right ophthalmic arteries [Figure 5g and h]. The patient was treated with embolization of the main feeders and his vision improved.
Visual impairment due to venous infarcts in the occipital cortex
Cortical blindness due to damage to the geniculocalcarine tract and the visual cortex is usually caused by bilateral PCA occlusion. Rarely, cortical blindness can be the initial manifestation of CVT.[31]
Case 6 [Figure 6]
Figure 6.
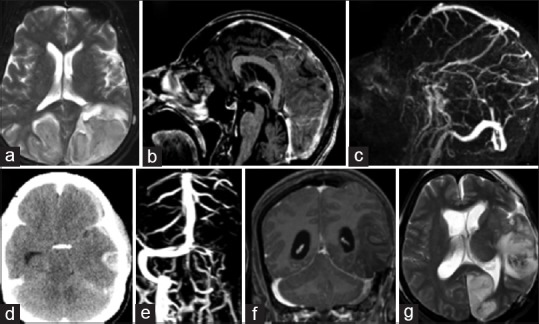
(a) T2 images showing large hemorrhagic venous infarcts in bilateral parietal-occipital lobes (b) magnetic resonance venography gado (c) TI gado showing filling defect posterior superior sagittal sinus. (d) Plain computerized tomography scan showing hemorrhagic venous infarct in the left temporal lobe with mass effect and midline shift wit effacement of the ventricles. (e and f) Magnetic resonance imaging postleft fronttemporoparietal decompressive craniectomy - magnetic resonance venography gado and TI gado show thrombosis of the left transverse and sigmoid sinuses (g) left occipital arterial infarcts secondary to arterial (posterior cerebral artery) compression
A 63-year-old male who was on treatment for diabetes, hypertension, hypothyroidism, and Parkinson's disease presented with an acute confusional state following an afternoon nap. On waking up, the patient was not sure whether it was daytime or night. He kept insisting that he was seeing a child falling down. His wife also noticed patient could not notice her presence from the left side. The patient disregarded a cup of tea kept in front of him and repeatedly asked for a tumbler of tea. Over the next 12 h, his sensorium gradually brought to the hospital. His Glasgow Coma Scale was found to be low 12/15 (E4V3M5) pupils bilaterally reactive to light and accommodation, and there was a left homonymous hemianopia. The MRI/MRV showed large hemorrhagic venous infarcts involving bilateral parietooccipital lobes with filling defect in the mid and posterior thirds of the SSS [Figure 6a–c].
Occipital venous infarcts presenting with complex visual hallucinations can be a very rare presentation. Subasree et al.[32] had reported a case of CVT presenting with features of Charles Bonnet syndrome due to venous congestion caused by CVT.
In these cases, standard treatment followed for venous infarcts using UFH or subcutaneous LMWH to attempt recanalization, antiepileptic agents, and drugs to reduce the ICPs caused by the edema of the venous infarcts.
Visual impairment due to arterial infarcts in the occipital cortex in large venous infarcts with mass effects
Case 7 [Figure 6d–f] (occipital arterials infarcts)
A 31-year-old female who presented with acute severe holocranial headache, giddiness, and irrelevant speech followed by an episode of generalized tonic–clonic seizures. Imaging with CT and MRI showed a hemorrhagic venous infarct in left temporal lobe with mass effect [Figure 6d–f]. She was taken up immediately for a left frontotemporoparietal decompressive craniectomy. The postsurgical period was uneventful, and she was noted to have deficit in the right half of the visual field. A follow-up MRI showed left occipital arterial [Figure 6g] infarct.
Patients with impending herniation due to large hemispheric venous infarction will require decompressive hemicraniectomy[33] rarely with large venous infarcts involving the temporal lobe; herniation can cause compression of the PCA against tentorium resulting in secondary arterial occipital lobe infarcts.[34]
CONCLUSIONS
Here, we have illustrated the various mechanisms causing visual impairment in patients with CVT and how they can be best managed. We have highlighted the importance of close monitoring in the acute phase (cases 1 and 4) as well as in the follow-up phase (case No. 5) since these patients can develop fresh complications through a different pathophysiological mechanism requiring prompt action with a different treatment strategy. There is a lack of literature on this subject, especially on the choice, timing, efficacy, and complications of these various treatment modalities such as ONSF and theco-peritoneal shunts. Future studies should give us more clarity and identify the best treatment to be offered for these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F ISCVT Investigators. Prognosis of cerebral vein and Dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–70. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 2.Barnett HJ, Hyland HH. Noninfective intracranial venous thrombosis. Brain. 1953;76:36–49. doi: 10.1093/brain/76.1.36. [DOI] [PubMed] [Google Scholar]
- 3.Ferro JM, Lopes MG, Rosas MJ, Ferro MA, Fontes J Cerebral Venous Thrombosis Portugese Collaborative Study Group. Long-term prognosis of cerebral vein and dural sinus thrombosis. Results of the VENOPORT study. Cerebrovasc Dis. 2002;13:272–8. doi: 10.1159/000057855. [DOI] [PubMed] [Google Scholar]
- 4.Nithyanandam S, Bhargava M. Visual Loss and Associated Ocular Manifestations of Cerebral Venous Thrombosis. AIOC 2008 Proceedings. 2008:360–1. [Google Scholar]
- 5.Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53:1537–42. doi: 10.1212/wnl.53.7.1537. [DOI] [PubMed] [Google Scholar]
- 6.Daif A, Awada A, al-Rajeh S, Abduljabbar M, al Tahan AR, Obeid T, et al. Cerebral venous thrombosis in adults. A study of 40 cases from Saudi Arabia. Stroke. 1995;26:1193–5. doi: 10.1161/01.str.26.7.1193. [DOI] [PubMed] [Google Scholar]
- 7.Lin A, Foroozan R, Danesh-Meyer HV, De Salvo G, Savino PJ, Sergott RC. Occurrence of cerebral venous sinus thrombosis in patients with presumed idiopathic intracranial hypertension. Ophthalmology. 2006;113:2281–4. doi: 10.1016/j.ophtha.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 8.Boulton M, Armstrong D, Flessner M, Hay J, Szalai JP, Johnston M. Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol. 1998;275(3 Pt 2):R889–96. doi: 10.1152/ajpregu.1998.275.3.R889. [DOI] [PubMed] [Google Scholar]
- 9.Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Prog Retin Eye Res. 2016;50:108–44. doi: 10.1016/j.preteyeres.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tso MO, Hayreh SS. Optic disc edema in raised intracranial pressure. IV. Axoplasmic transport in experimental papilledema. Arch Ophthalmol. 1977;95:1458–62. doi: 10.1001/archopht.1977.04450080168023. [DOI] [PubMed] [Google Scholar]
- 11.Sadun AA, Currie JN, Lessell S. Transient visual obscurations with elevated optic discs. Ann Neurol. 1984;16:489–94. doi: 10.1002/ana.410160410. [DOI] [PubMed] [Google Scholar]
- 12.Meador KJ, Swift TR. Tinnitus from intracranial hypertension. Neurology. 1984;34:1258–61. doi: 10.1212/wnl.34.9.1258. [DOI] [PubMed] [Google Scholar]
- 13.Caffery TS, Perret JN, Musso MW, Jones GN. Optic nerve sheath diameter and lumbar puncture opening pressure in nontrauma patients suspected of elevated intracranial pressure. Am J Emerg Med. 2014;32:1513–5. doi: 10.1016/j.ajem.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Einhäupl KM, Villringer A, Meister W, Mehraein S, Garner C, Pellkofer M, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338:597–600. doi: 10.1016/0140-6736(91)90607-q. [DOI] [PubMed] [Google Scholar]
- 15.de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30:484–8. doi: 10.1161/01.str.30.3.484. [DOI] [PubMed] [Google Scholar]
- 16.Dentali F, Squizzato A, Gianni M, De Lodovici ML, Venco A, Paciaroni M, et al. Safety of thrombolysis in cerebral venous thrombosis. A systematic review of the literature. Thromb Haemost. 2010;104:1055–62. doi: 10.1160/TH10-05-0311. [DOI] [PubMed] [Google Scholar]
- 17.Choulakian A, Alexander MJ. Mechanical thrombectomy with the penumbra system for treatment of venous sinus thrombosis. J Neurointerv Surg. 2010;2:153–6. doi: 10.1136/jnis.2009.001651. [DOI] [PubMed] [Google Scholar]
- 18.Coutinho JM, Ferro JM, Zuurbier SM, Mink MS, Canhão P, Crassard I, et al. Thrombolysis or anticoagulation for cerebral venous thrombosis: Rationale and design of the TO-ACT trial. Int J Stroke. 2013;8:135–40. doi: 10.1111/j.1747-4949.2011.00753.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanley DF, Feldman E, Borel CO, Rosenbaum AE, Goldberg AL. Treatment of sagittal sinus thrombosis associated with cerebral hemorrhage and intracranial hypertension. Stroke. 1988;19:903–9. doi: 10.1161/01.str.19.7.903. [DOI] [PubMed] [Google Scholar]
- 20.Torikoshi S, Akiyama Y. Report of dramatic improvement after a lumboperitoneal shunt procedure in a case of anticoagulation therapy-resistant cerebral venous thrombosis. J Stroke Cerebrovasc Dis. 2016;25:e15–9. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Wang VY, Barbaro NM, Lawton MT, Pitts L, Kunwar S, Parsa AT, et al. Complications of lumboperitoneal shunts. Neurosurgery. 2007;60:1045–8. doi: 10.1227/01.NEU.0000255469.68129.81. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni GB, Sanehalli M, Ramakrishna S, Mustare V. Abstract TP76: Role of surgery in patients with cerebral venous sinus thrombosis with visual impairment. Stroke. 2016;47:ATP76. [Google Scholar]
- 23.Kamiryo T, Hamada J, Fuwa I, Ushio Y. Acute subdural hematoma after lumboperitoneal shunt placement in patients with normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 2003;43:197–200. doi: 10.2176/nmc.43.197. [DOI] [PubMed] [Google Scholar]
- 24.Acheson JF, Green WT, Sanders MD. Optic nerve sheath decompression for the treatment of visual failure in chronic raised intracranial pressure. J Neurol Neurosurg Psychiatry. 1994;57:1426–9. doi: 10.1136/jnnp.57.11.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sergott RC, Savino PJ, Bosley TM. Modified optic nerve sheath decompression provides long-term visual improvement for pseudotumor cerebri. Arch Ophthalmol. 1988;106:1384–90. doi: 10.1001/archopht.1988.01060140548021. [DOI] [PubMed] [Google Scholar]
- 26.Nithyanandam S, Manayath GJ, Battu RR. Optic nerve sheath decompression for visual loss in intracranial hypertension: Report from a tertiary care center in South India. Indian J Ophthalmol. 2008;56:115–20. doi: 10.4103/0301-4738.39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murdock J, Tzu JH, Schatz NJ, Lee WW. Optic nerve sheath fenestration for the treatment of papilledema secondary to cerebral venous thrombosis. J Neuroophthalmol. 2014;34:67–9. doi: 10.1097/WNO.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 28.Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland JJ. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry. 1998;65:308–16. doi: 10.1136/jnnp.65.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kühner A, Krastel A, Stoll W. Arteriovenous malformations of the transverse Dural sinus. J Neurosurg. 1976;45:12–9. doi: 10.3171/jns.1976.45.1.0012. [DOI] [PubMed] [Google Scholar]
- 30.Tsai LK, Jeng JS, Liu HM, Wang HJ, Yip PK. Intracranial Dural arteriovenous fistulas with or without cerebral sinus thrombosis: Analysis of 69 patients. J Neurol Neurosurg Psychiatry. 2004;75:1639–41. doi: 10.1136/jnnp.2003.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Moon SJ, Olivero WC, Wang H. Cortical blindness as a rare presentation of cerebral venous thrombosis. J Surg Case Rep 2013. 2013 doi: 10.1093/jscr/rjt035. pii: Rjt035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subasree R, Kulkarni GB, Kumar MV, Bharath RD, Sandhya M, Yadav R, et al. Charles bonnet syndrome in a case of cerebral venous thrombosis with fMRI-EEG correlation. Neurol India. 2014;62:557–9. doi: 10.4103/0028-3886.144489. [DOI] [PubMed] [Google Scholar]
- 33.Aaron S, Alexander M, Moorthy RK, Mani S, Mathew V, Patil AK, et al. Decompressive craniectomy in cerebral venous thrombosis: A single centre experience. J Neurol Neurosurg Psychiatry. 2013;84:995–1000. doi: 10.1136/jnnp-2012-303356. [DOI] [PubMed] [Google Scholar]
- 34.Keane JR. Blindness following tentorial herniation. Ann Neurol. 1980;8:186–90. doi: 10.1002/ana.410080209. [DOI] [PubMed] [Google Scholar]