Figure 1. Akt3 is a first-responding isozyme to reactive native lipid signals.
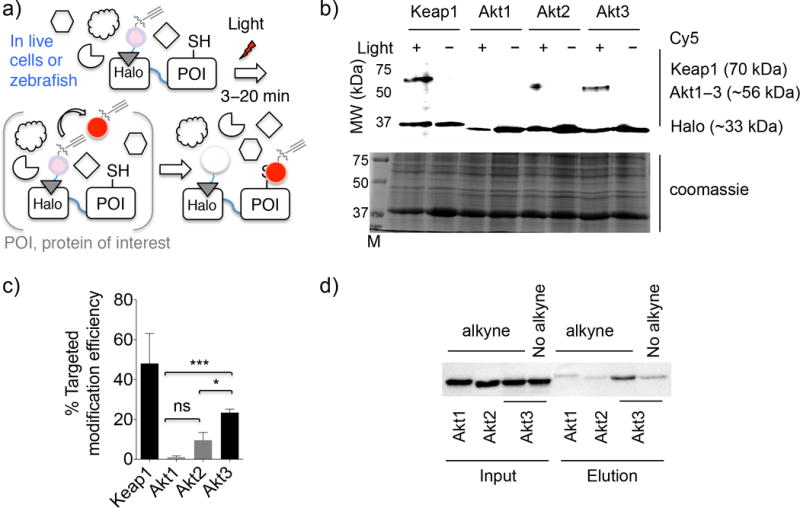
(a) T-REX on-demand redox targeting involves light-driven liberation of LDE signal (red dot) in substoichiometric amounts from a photocaged precursor covalently bound to HaloTag. Class II proximity enhancement7 enables targeted covalent modification of protein of interest (POI; genetically fused to HaloTag) by specific LDEs. Light source: 365 nm, 0.3 mW/cm2 hand-held UV-lamp placed at 1-inch above samples (3–20 min in cells15 or fish embryos). (b) T-REX screen (Supplementary Fig. 1) and validation identified Akt3 to be a first HNE-responder. Keap1 was used for comparison. Top: Cy5 channel; Bottom: Cy5 signal from samples treated with or without light, followed by TEV-protease treatment. M designates MW (molecular weight)-ladder. See Supplementary Fig. 2a for full gels. (c) Quantitation: the Cy5 signal intensity on the band corresponding to POI MW in the samples exposed to light was normalized by the signal intensity on Halo on the corresponding samples not exposed to light. Error bars designate s.d. (Keap1, n =4; Akt1, Akt2, and Akt3, n = 3 independent biological replicates). (d) Orthogonal validation using Click coupling with biotin-azide followed by streptavidin enrichment subsequent to T-REX-enabled targeted-HNE(alkyne)-modification in live cells. “No alkyne” corresponds to probe that had no-alkyne functionalization (Supplementary Fig. 1b), controlling for any non-specific binding/biotinylation. See Supplementary Fig. 2b for a full blot.
