Figure 2. C119 of Akt3 is the unique HNE-sensing residue.
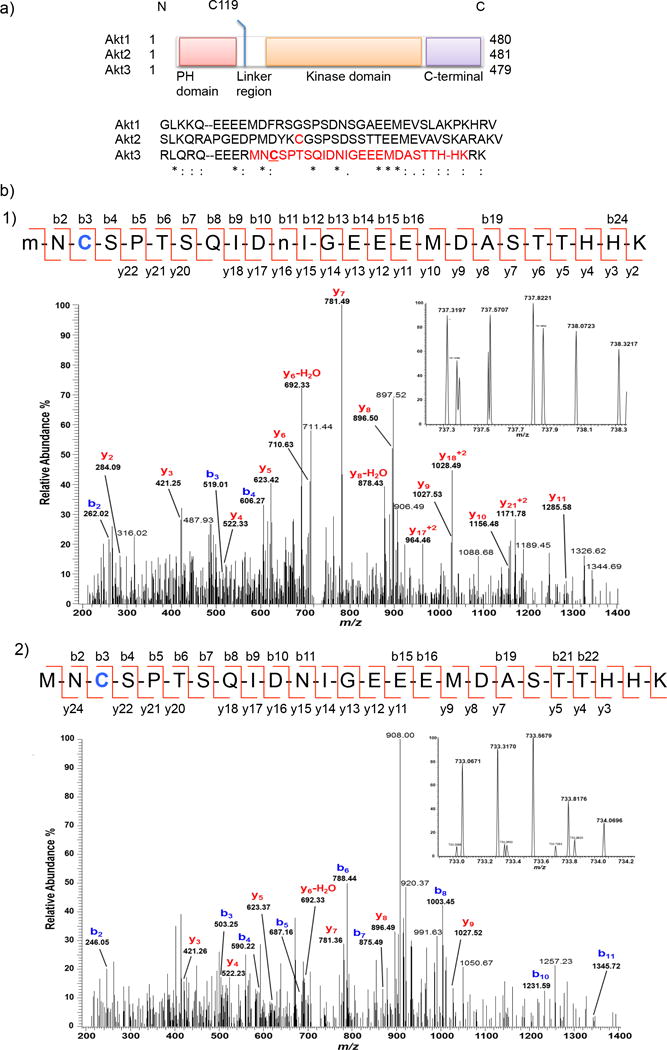
(a) Domain composition of Akt isoforms: the linker region displays the highest divergence in amino acid sequence (shown) among the three isozymes. C124 of Akt2 (in red) is sensitive to H2O219. We identified C119 (underlined) of Akt3 to be the site of HNE(alkyne)-modification on the tryptic peptide shown in red (also see Supplementary Fig. 3). (b) MS/MS Spectra of quadruply-charged ions at m/z 737.31974+ (Panel-1) and m/z 737.06714+ (Panel-2) identify an HNE-modified peptide at C119 residue from Akt3 protein. Although y-ion series in the spectra do not cover the C119 modification, the b-ion series in each MS/MS spectrum along with the high accurate mass (<5 ppm) of the precursor ion (see inset for the MS spectrum) provide a confident identification of C119 modification with reduced HNE-alkyne (+154.1 Da). An additional oxidation on M1 residue and a deamidation on N11 residue (indicated by lower-case m and n) were identified in the peptide (Panel-1). See Supplementary Table 2–3.
