Figure 4. Akt3(C119)-Specific HNEylation upregulates transcriptional activities of tumor suppressor genes.
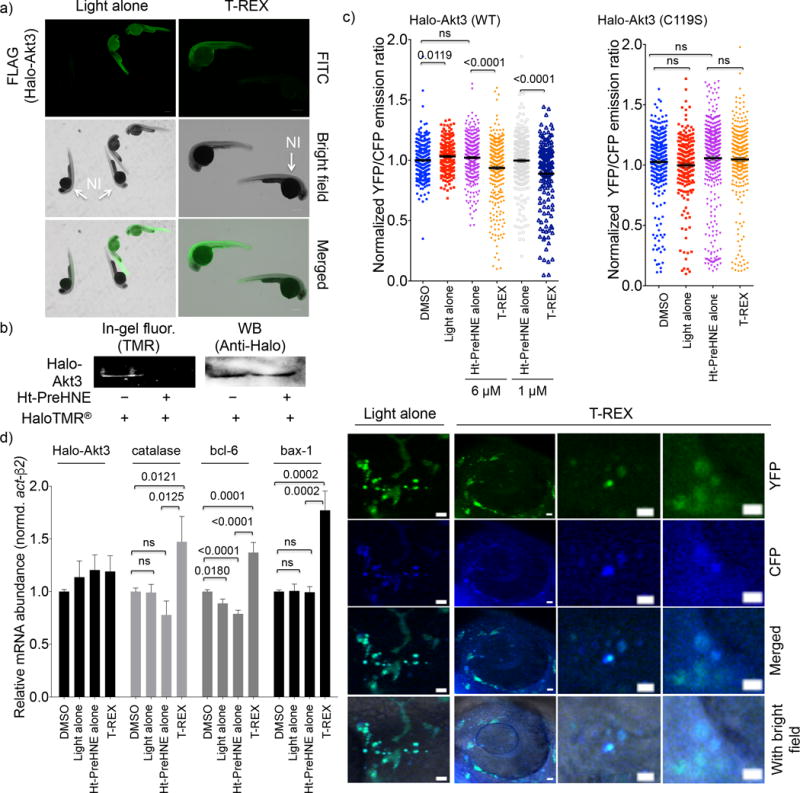
(a) Whole-mount immunofluorescence, detecting for FLAG in fish injected with Halo-Akt3(FLAGx2) mRNA. The non-injected (NI), untreated fish are shown as control. Scale bar, 300 μm. Also see Supplementary Fig. 11. (b) Fish injected with Halo-Akt3 mRNA express the full-length fusion protein (~90 kDa). Pre-treatment of whole embryos with Ht-PreHNE prevents labeling of Halo-Akt3 with HaloTMR® post lysis. Also see Supplementary Fig. 13a for a full gel and blot. (c) Quantitation (Upper) and representative images (lower) of CFP and YFP fluorescence in fish co-injected with Halo-Akt3 mRNA and AktAR plasmid DNA. Also see Supplementary Fig. 15–17. Scale bar: 10 μm. YFP/CFP ratiometric image quantification of individual somatic cells [297 (DMSO), 230 (light alone), 315/270 (Ht-PreHNE alone, 6 vs. 1 μM), and 325/168 (T-REX, 6 vs. 1 μM)] cells from n=5 independent embryos] indicates Akt3-C119-specific HNEylation in wt-fish embryos enabled by T-REX (Supplementary Fig. 16–17) downregulates the kinase activity; these effects were ablated in Akt3(C119S) mutant [298 (DMSO), 225 (light alone), 422 (Ht-PreHNE alone, 1 μM), and 317 (T-REX)]. Error bars are s.e.m. (d) Consistent with results in (c), since Akt3 is an established upstream antagonist of FOXO and p53, Akt3-specific HNEylation-dependent kinase-activity downregulation upregulates transcriptional activities of FOXO and p53 tumor suppressors. Catalase and Bcl6, and Bax-1 are driven by FOXO and p53, respectively. Error bars designate s.e.m (n = 6 independent biological replicates and two technical repeats for each bar). These changes were not observed for Halo-Akt3(C119S) or Halo-Akt2 (Supplementary Fig. 18).
