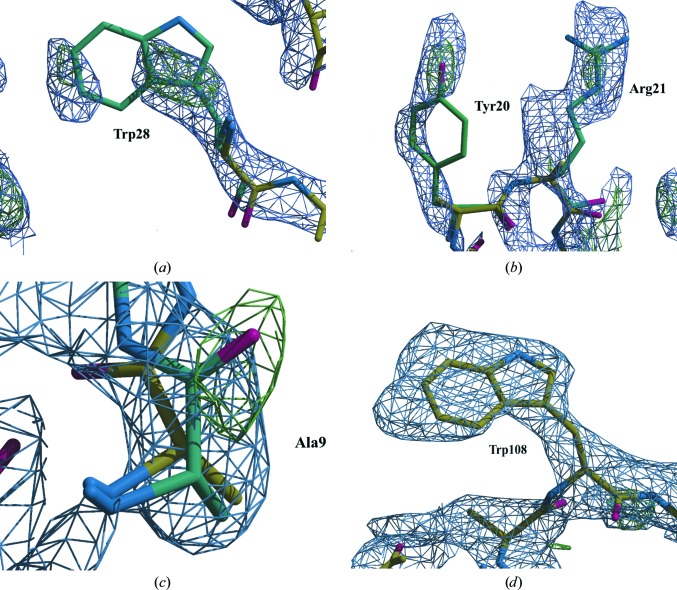
Figure 5.
Automated model building using the single-crystal data. After molecular replacement with the polyalanine monomer (yellow C atoms), the difference map shows the position of bulky side-chain residues such as (a) Trp28 as placed during autobuilding by Buccaneer (turquoise C atoms) and (b) Tyr20 and Arg21. The map is stretched, which is typical for incomplete data; as always with poor map quality, careful interpretation of the region is required. The map improves after side-chain reconstruction with Buccaneer and refinement with REFMAC5. (c) The refined density suggests that Ala9 (yellow C atoms) is a cis-peptide; it is confirmed by the X-ray structure of the same polymorph (turquoise C atoms; PDB entry 4r0f) that the peptide is cis. Refinement using standard protocols can further improve the map and shows continuous density (d) for a Trp108 side-chain residue in chain A of the single-crystal model. All density is shown at a standard contour level of 1.2σ.