Abstract
Background
Few published studies have evaluated the power of the oxygen uptake efficiency slope (OUES) to predict outcomes in patients with idiopathic pulmonary arterial hypertension (IPAH), who typically die of right‐sided heart failure. Our study sought to evaluate the power of OUES to predict clinical worsening and mortality in patients with IPAH.
Methods and Results
Patients with newly diagnosed IPAH who underwent symptom‐limited cardiopulmonary exercise testing from November 11, 2010, to June 25, 2015, in our hospital were prospectively enrolled and followed for up to 66 months. Clinical worsening and mortality were recorded. A total of 210 patients with IPAH (159 women; mean age, 32±10 years) were studied with a median follow‐up of 41 months. Thirty‐one patients died, 1 patient underwent lung transplantation, and 85 patients presented with clinical worsening. The univariate analysis revealed that OUES, OUESI (OUESI=OUES/body surface area), peak oxygen uptake (), peak , ventilation ()/carbon dioxide output () slope, peak systolic blood pressure, heart rate recovery, pulmonary vascular resistance, cardiac index, N‐terminal prohormone brain natriuretic peptide, and World Health Organization functional class were all predictive of clinical worsening and mortality (all P<0.05). Multivariate analysis demonstrated that OUESI and cardiac index were independently predictive of clinical worsening, and OUESI and N‐terminal prohormone brain natriuretic peptide were independently predictive of mortality. Patients with OUESI ≤0.52 m−2 had a worse 5‐year survival rate than patients with OUESI >0.52 m−2 (41.9% versus 89.8%, P<0.0001).
Conclusions
The OUES, a submaximal parameter obtained from cardiopulmonary exercise testing, provides prognostic information for predicting clinical worsening and mortality in patients with IPAH.
Keywords: cardiopulmonary exercise testing, idiopathic pulmonary arterial hypertension, oxygen uptake efficiency slope
Subject Categories: Pulmonary Hypertension
Clinical Perspective
What is New?
In this study, we demonstrated that oxygen uptake efficiency slope, a submaximal parameter, is more strongly associated with clinical worsening and all‐cause mortality than minute ventilation/carbon dioxide output slope, and peak oxygen consumption in patients with idiopathic pulmonary arterial hypertension.
What are the Clinical Implications?
Oxygen uptake efficiency slope appears to be one of the strongest prognostic markers among exercise variables obtained from cardiopulmonary exercise testing in patients with idiopathic pulmonary arterial hypertension.
Introduction
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by increased remodeling of pulmonary vasculature that leads to right‐sided heart failure and death. It is important to identify patients with a higher risk of poor outcome early in order to treat them with optimal drugs, such as initial combination therapy for patients with a higher risk of mortality.1 Several key parameters obtained from cardiopulmonary exercise testing (CPET), including peak oxygen uptake (peak ), end tidal partial pressure of carbon dioxide at anaerobic threshold, ventilation ()/carbon dioxide output () slope or ratio at anaerobic threshold, and heart rate recovery have been widely used to assess disease severity, therapeutic responses, and prognosis estimation in patients with PAH.2, 3
In recent years, the oxygen uptake efficiency slope (OUES), representing the rate of increase in oxygen uptake () in response to a 10‐fold increase in ventilation () during incremental exercise (), has been introduced by Baba et al.4, 5 The advantages of OUES are that it can be calculated from submaximal exercise data and it is stable even when 50%, 75%, or 100% of the exercise data are used for the calculation. In addition, it is independent of interobserver and intraobserver variability.6
Tan et al7 demonstrated that OUES was significantly lower in patients with idiopathic PAH (IPAH) compared with that in healthy controls. Woods et al8 showed that OUES was able to differentiate between patients with mild and severe pulmonary hypertension.8 Only one study, with a relatively small number of patients, has shown that OUES was a better predictor than slope and peak and could predict poor outcome in 98 patients with PAH. Among them, 48 patients had IPAH and 50 patients had PAH with associated conditions.9 It is still controversial whether OUES is better than the slope for predicting adverse events in patients with left‐sided heart failure.10, 11 Recently, time to clinical worsening (CW) has become a better primary end point for randomized controlled trials in patients with PAH12, 13; however, no study has evaluated the value of OUES in predicting CW in patients with IPAH. Therefore, the aim of this study was to assess the prognostic value of OUES for CW and mortality in patients with IPAH.
Methods
Study Participants
Consecutive adult patients with newly diagnosed IPAH admitted to Fuwai Hospital were prospectively enrolled from November 11, 2010, to June 25, 2015. IPAH was defined according to the 2009 European Society of Cardiology/European Respiratory Society guideline for the diagnosis and treatment of pulmonary hypertension.14 Patients who were unable to perform an exercise test or had contraindications to exercise testing were excluded. Basic demographics, medications, hemodynamic measurements from right‐sided heart catheterization, and World Health Organization functional class (WHO‐FC) were obtained from the medical records. This study complies with the Declaration of Helsinki and was approved by the institutional review board. Written informed consent was obtained from all participants.
Cardiopulmonary Exercise Testing
Symptom‐limited CPET was performed using the COSMED Quark CPET system on all recruited patients with IPAH at baseline, before they received specific drug therapy. All patients rested for 3 minutes followed by 3 minutes of unloaded pedaling and exercise using a progressively increasing work rate of 5 to 20 W·min−1 (the rate of increasing work rate depended on the estimated exercise capacity of each patient) to a maximum tolerance on an electromagnetically braked cycle ergometer. Cardiac rhythm, measured by a standard 12‐lead ECG, and oxyhemoglobin saturation were continuously recorded. Heart rate was recorded at 1‐minute intervals. Blood pressure was measured every 3 minutes and at the peak of the exercise. The test was performed by experienced medical staff, and the equipment was calibrated before each test.
Calculation of CPET Measures
Gas exchange variables were measured by a metabolic cart (COSMED) on a breath‐by‐breath basis and averaged over 10‐second intervals. Peak was defined as the highest 30‐second average of oxygen consumption in the last minute of exercise. Other peak values were also calculated at the same time point. Anaerobic threshold was determined by the V‐slope method and corroborated using other plots. Peak /heart rate was calculated as peak divided by peak heart rate. Since previous studies show that slope calculated using the whole exercise period, as opposed to from the start of exercise to the ventilatory compensation point in patients with left‐sided heart failure and PAH, has better prognostic value,11, 15, 16 slope was determined by linear regression using the whole exercise period. OUES was determined by the slope of the regression line between log10 minute ventilation (x axis, L·min−1) and (y axis, L·min−1) during the whole exercise period (). A higher OUES (steeper slope) represents more efficient oxygen being delivered in the body; while a lower OUES (more flat slope) represents a higher amount of ventilation required in response to a given oxygen uptake (Figure 1A and 1B).4 Predicted values for OUES were calculated from the equations introduced by Hollenberg and Tager17 In men, OUES=[1320−(26.7×age)+(1394×body surface area)]/1000 and, in women, OUES=[1175−(15.8×age)+(841×body surface area)]/1000. Percent predicted peak was calculated based on normative values proposed by Hansen and Wasserman et al.18, 19 Heart rate recovery was calculated as maximum heart rate‐postexercise heart rate after 2 minutes during the recovery period.
Figure 1.
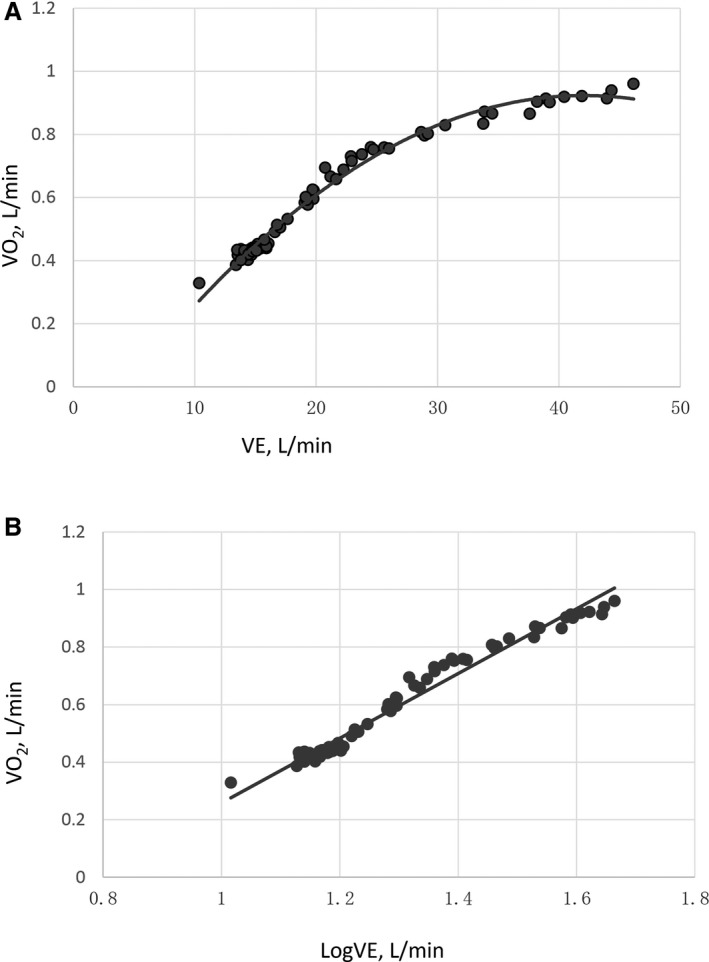
The relationship between and during incremental exercise in a 58‐year‐old woman with idiopathic pulmonary arterial hypertension. Linear (A) and semi‐log (x axis) plots of the data (B) are presented.
Other Measures
Right‐sided heart catheterization with standard hemodynamic measurements was performed at baseline within 3 days of each patient's CPET study, as we previously reported.20 NT‐proBNP (N‐terminal prohormone brain natriuretic peptide) was determined at baseline using an enzyme immunoassay kit (Biomedica Medizinprodukte GmbH&CoKG).
Follow‐Up
Patients were followed‐up every 3 months for 1 year, then every 6 months after they were discharged. WHO‐FC, medications and side effects, the date and cause of lung transplantation, and death were documented at each follow‐up. The end point of mortality was defined as all‐cause mortality or lung transplantation. The end point of CW was defined as the time from CPET to the first event, which included the following: all‐cause mortality, lung transplantation, hospitalization for worsening of PAH, the need for epoprostenol therapy, and interventional procedures (performance of balloon atrial septostomy).12, 21 If patients experienced CW before death or lung transplantation, they were included in the analyses of both mortality and CW. The follow‐up time was calculated from the date of CPET to the end point date or May 1, 2016.
Statistical Analysis
Continuous variables were expressed as mean±SD and categorical variables as a number or percentage as appropriate. Comparisons between groups were performed using independent‐samples Student t tests or 1‐way ANOVA for normally distributed variables and Mann–Whitney U test for non‐normally distributed variables. Chi‐square test was used for categorical variables. Multiple linear regression analysis (stepwise) was performed to assess the association between OUES and independent variables, which included age, sex, height, weight, body surface area (BSA), and body mass index. Correlations between 2 variables were explored using either the Pearson or Spearman correlation coefficient as appropriate.
A Cox proportional hazards regression analysis was used to detect predictors associated with CW and survival. The variables predictive of CW and survival by univariate analysis (P<0.05) were then entered into a multivariable Cox regression analysis with a forward procedure (likelihood ratio) to determine independent predictors of CW and survival. Since there were not enough events, in order to avoid overfitting, for CW, we put only WHO‐FC, NT‐proBNP, pulmonary vascular resistance (PVR) cardiac index, peak , OUESI, slope, heart rate recovery, and peak systolic blood pressure into the multivariable forward stepwise Cox analysis, and for mortality, we put only NT‐proBNP, PVR, peak , OUESI, and slope into the multivariable forward stepwise Cox analysis, considering both P values of the variable in univariate Cox analysis and their clinical significance.
Receiver operator characteristic curve analysis was used to identify the cutoff point for parameters associated with CW and survival, using data censored to 10 months (the shortest duration of follow‐up in our study). Kaplan–Meier survival charts were created to determine differences in the rate of cardiac events between patients with values above or below the cutoff point. Curves were compared using the log‐rank test. A 2‐sided P<0.05 was considered statistically significant. Receiver operator characteristic curve analysis was performed using MedCalc for Windows, version 13.0 (MedCalc Software), and other statistical analyses were performed using SPSS version 19.0 (SPSS, Inc).
Results
Baseline Characteristics
A total of 213 patients were identified with IPAH and underwent CPET. Three patients were lost to follow‐up and not included in the analysis. The mean age was 32±10 years, and 75.7% of patients were women. A total of 112 patients presented with WHO‐FC I and II, and 98 patients presented with WHO‐FC III and IV. The mean pulmonary vascular resistance was 12.5±6.3 Wood units and the mean cardiac index was 2.8±0.9 L·min−1·m−2. Anaerobic threshold could not be identified in 17 patients. Twenty‐one patients were treated with calcium channel blockers, 182 patients were treated with monotherapy (7 patients with prostacyclins, 33 patients with endothelin receptor antagonists, and 142 patients with phosphodiesterase type 5 inhibitors), and 7 patients were treated with combination therapy. The median duration of follow‐up was 41±15 months. During the follow‐up period, 31 patients died, 1 patient underwent lung transplantation, and 85 patients presented with CW.
Differences in Variables Between Groups
Patients with subsequent CW had a similar sex distribution, age, BSA, and body mass index compared with patients without subsequent CW (non‐CW). However, patients with subsequent CW had a poorer WHO‐FC, higher NT‐proBNP, and much more severe hemodynamic parameters. In addition, patients who presented with CW had significantly lower peak , OUES, end tidal partial pressure of carbon dioxide at anaerobic threshold, heart rate recovery, and peak systolic blood pressure, along with higher slope. There was no difference in the peak respiratory exchange ratio between patients with and without subsequent CW. These results also apply to the comparison between survivors and nonsurvivors (Table 1).
Table 1.
Demographic and Clinical Data of CW vs Non‐CW and Survivors vs Nonsurvivors in Patients With IPAH
| Variables | CW (n=85) | Non‐CW (n=125) | Nonsurvivors (n=32) | Survivors (n=178) |
|---|---|---|---|---|
| Age, y | 32.8±11.2 | 31.9±9.9 | 33.9±14.4 | 32.0±9.6 |
| Women, % (No.) | 74.1 (63) | 76.8 (96) | 65.6 (21) | 77.5 (138) |
| BSA, m2 | 1.64±0.15 | 1.64±0.17 | 1.64±0.14 | 1.64±0.17 |
| BMI, kg·m−2 | 22.4±3.3 | 22.6±3.6 | 22.0±3.3 | 22.6±3.5 |
| WHO‐FC, No. | ||||
| I or II | 35 | 77a | 10 | 102a |
| III or IV | 50 | 48 | 22 | 76 |
| NT‐proBNP, pg/mL | 1669.5±1052.7 | 1052.4±800.1b | 1172.1±821.4 | 2026.0±1301.7b |
| Mono/combination therapy, n | 81/4 | 121/3 | 30/2 | 173/5 |
| Hemodynamics | ||||
| RAP, mm Hg | 6.4±5.0 | 4.5±3.9a | 7.7±5.4 | 4.8±4.1a |
| mPAP, mm Hg | 62.1±15.7 | 55.7±16.3b | 65.4±16.2 | 57.0±16.1a |
| Cardiac index, L·min−1·m−2 | 2.4±0.6 | 3.0±1.0b | 2.4±0.7 | 2.9±0.9c |
| PVR, Wood units | 15.0±6.7 | 10.9±5.4b | 15.8±8.2 | 12.0±5.7a |
| CPET | ||||
| Peak , mL·min−1 | 669.2±186.8 | 828.6±250.6b | 647.0±166.7 | 785.1±245.0a |
| Peak , mL·kg−1·min−1 | 11.2±2.6 | 13.9±3.6b | 11.0±2.5 | 13.1±3.6b |
| Peak , % predicted | 33.2±10.1 | 40.8±11.6b | 31.8±9.4 | 38.8±11.7b |
| AT, mL·kg−1·min−1 | 9.2±2.3 | 10.2±2.5a | 9.1±2.4 | 9.9±2.5 |
| OUES | 0.79±0.22 | 1.04±0.33b | 0.78±0.19 | 0.97±0.32b |
| OUESI, m−2 | 0.48±0.12 | 0.63±0.18b | 0.46±0.10 | 0.59±0.18b |
| OUES, % predicted | 36.3±10.7 | 47.2±14.7b | 34.7±9.5 | 44.3±14.5b |
| slope | 55.8±20.0 | 43.4±13.8b | 58.1±19.1 | 46.7±16.8b |
| PETCO2@AT, mm Hg | 26.3±5.4 | 30.5±4.9b | 25.9±5.1 | 29.3±5.4b |
| Peak /heart rate, mL·min−1·beat−1 | 5.0±1.4 | 5.8±1.7b | 5.0±1.6 | 5.6±1.6c |
| Peak work rate, W | 62.0±22.3 | 77.8±25.0b | 63.4±26.6 | 72.9±24.7 |
| Peak RER | 1.11±0.24 | 1.11±0.12 | 1.16±0.37 | 1.10±0.11 |
| Peak heart rate, min−1 | 136.5±20.3 | 146.1±20.2a | 134.1±26.4 | 143.7±19.3 |
| HRR, min−1 | 17.9±11.2 | 23.9±14.7b | 15.2±12.6 | 22.6±13.7a |
| Peak SBP, mm Hg | 116.8±26.0 | 135.5±36.1b | 110.5±18.3 | 131.1±34.8b |
| Peak DBP, mm Hg | 82.8±19.6 | 86.4±21.8 | 77.7±12.7 | 86.2±21.9c |
Data are presented as percentage (number) or mean±SD. AT indicates anaerobic threshold; BMI, body mass index; BSA, body surface area; CPET, cardiopulmonary exercise testing; CW, clinical worsening; DBP, diastolic blood pressure; HRR, heart rate recovery; mPAP, mean pulmonary arterial pressure; NT‐proBNP, N‐terminal prohormone brain natriuretic peptide; OUES, oxygen uptake efficiency slope; OUESI, oxygen uptake efficiency slope index (OUES/BSA); PETCO2@AT, end tidal partial pressure of carbon dioxide at anaerobic threshold; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RER, respiratory exchange ratio; SBP, systolic blood pressure; , carbon dioxide output; , minute ventilation; , oxygen consumption; WHO‐FC, World Health Organization functional class.
P<0.01.
P<0.001.
P<0.05.
Oxygen Uptake Efficiency Slope
Multivariate linear regression analysis showed that OUES only increased linearly with BSA (R=0.40, P<0.0001), therefore we defined the OUES index (OUESI) as OUES divided by BSA. The mean value of OUES was 0.94±0.31 and the mean value of OUESI was 0.57±0.171 m−2. The mean values of OUES and OUESI were significantly different among the different WHO‐FCs (all P<0.0001; Figures 2 and 3). Predicted values for OUES were calculated according to the equations of Hollenberg and Tager. The mean predicted value of OUES was 2.23±0.49 and mean percent predicted OUES was 42.8±14.2%. The mean OUES in men was higher than that in women (1.04±0.39 versus 0.90±0.28, P<0.02). OUES was significantly correlated with peak (r=0.71, P<0.0001), slope (r=−0.67, P<0.0001), cardiac index (r=0.36, P<0.0001), and PVR (r=−0.55, P<0.0001).
Figure 2.
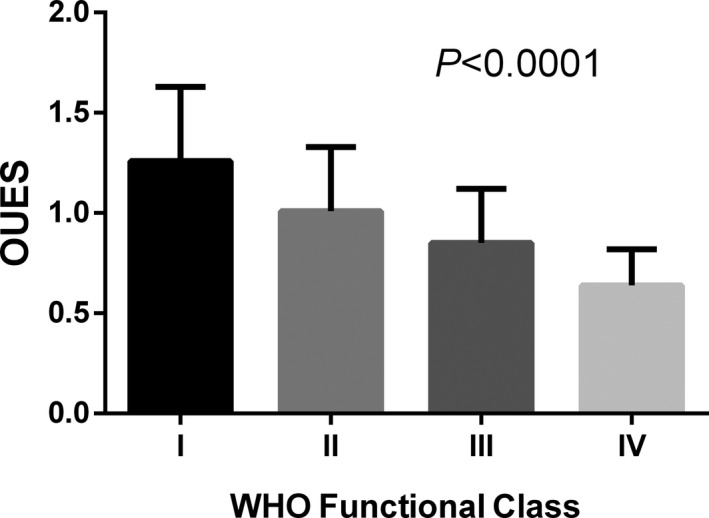
Mean values of oxygen uptake efficiency slope (OUES) divided into World Health Organization (WHO) functional classes.
Figure 3.
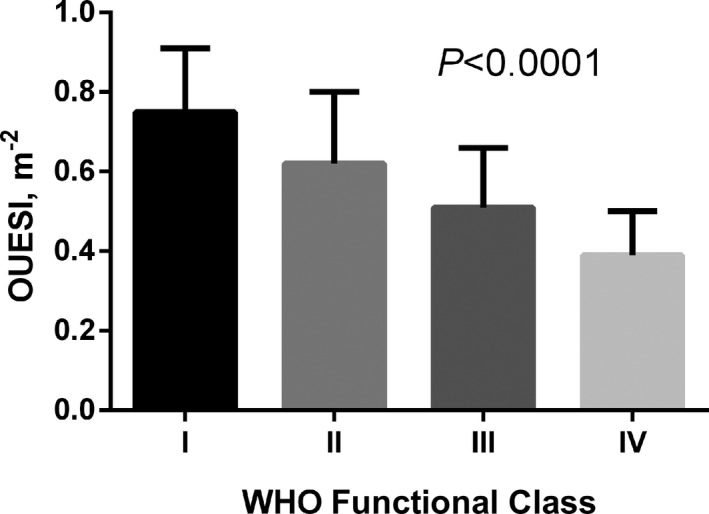
Mean values of oxygen uptake efficiency slope index (OUESI) divided into World Health Organization (WHO) functional classes.
Predictors for Long‐Term Outcomes
On univariate analysis, WHO‐FC, NT‐proBNP, and variables obtained from right‐sided heart catheterization and CPET, except for age and sex, were all significant predictors of CW and mortality (Table 2). Multivariable forward stepwise Cox analysis was performed including WHO‐FC, NT‐proBNP, PVR, cardiac index, peak , OUESI, slope, heart rate recovery, and peak systolic blood pressure. Results showed that OUESI (χ2: 26.54; hazard ratio [HR], 0.99 [95% CI, 0.99–0.99], P<0.0001) and cardiac index (χ2: 7.23; HR, 0.62 [95% CI, 0.44–0.88], P<0.007) were independently predictive of CW. The forward stepwise and backward stepwise analysis yielded the same results. With respect to mortality, because of the occurrence of only 32 events, we put only NT‐proBNP, PVR, peak , OUESI, and slope into the multivariable forward stepwise Cox analysis. The results showed that NT‐proBNP (χ2: 9.88; HR, 1.001 [95% CI, 1.000–1.001], P<0.002) and OUESI (χ2: 8.66; HR, 0.99 [95% CI, 0.99–0.99], P<0.003) were independently predictive of mortality. The forward stepwise and backward stepwise analyses yielded the same results.
Table 2.
Univariate Cox Analysis of Proportional Risks for Clinical Worsening and All‐Cause Mortality in Patients With IPAH
| Variables | Clinical Worsening | Mortality | ||||
|---|---|---|---|---|---|---|
| χ2 | HR (95% CI) | P Value | χ2 | HR (95% CI) | P value | |
| Age | 0.003 | 1.00 (0.98–1.02) | 0.957 | 0.59 | 1.01 (0.98–1.04) | 0.44 |
| Sex | 0.35 | 1.08 (0.84–1.37) | 0.552 | 2.18 | 0.58 (0.28–1.20) | 0.14 |
| WHO‐FC | 12.09 | 1.84 (1.31–2.59) | <0.0001 | 10.32 | 2.51 (1.43–4.41) | <0.002 |
| NT‐proBNP | 21.66 | 1.001 (1.000–1.001) | <0.0001 | 22.22 | 1.001 (1.000–1.001) | <0.0001 |
| RAP | 9.08 | 1.08 (1.03–1.13) | <0.003 | 9.13 | 1.11 (1.04–1.18) | <0.003 |
| mPAP | 8.65 | 1.02 (1.01–1.03) | <0.0001 | 6.48 | 1.02 (1.00–1.04) | <0.01 |
| Cardiac index | 21.38 | 0.46 (0.33–0.64) | <0.0001 | 5.46 | 0.55 (0.33–0.91) | <0.02 |
| PVR | 25.08 | 1.07 (1.04–1.10) | <0.0001 | 9.50 | 1.06 (1.02–1.11) | <0.002 |
| Peak | 28.40 | 0.99 (0.99–0.99) | <0.0001 | 11.13 | 0.99 (0.99–0.99) | <0.001 |
| Peak | 34.65 | 0.80 (0.74–0.86) | <0.0001 | 12.28 | 0.80 (0.71–0.91) | <0.0001 |
| Peak , % predicted | 26.73 | 0.94 (0.92–0.96) | <0.0001 | 11.24 | 0.94 (0.90–0.97) | <0.001 |
| AT | 10.24 | 0.87 (0.80–0.95) | <0.0001 | 4.03 | 0.87 (0.76–0.99) | <0.045 |
| OUES | 37.09 | 0.99 (0.99–0.99) | <0.0001 | 13.92 | 0.99 (0.99–0.99) | <0.0001 |
| OUESI | 41.91 | 0.99 (0.99–0.99) | <0.0001 | 15.84 | 0.99 (0.99–0.99) | <0.0001 |
| OUES, % predicted | 36.40 | 0.95 (0.93–0.97) | <0.0001 | 14.45 | 0.95 (0.92–0.97) | <0.0001 |
| slope | 26.03 | 1.02 (1.01–1.03) | <0.0001 | 10.26 | 1.02 (1.00–1.04) | <0.001 |
| PETCO2@AT | 29.11 | 0.90 (0.87–0.94) | <0.0001 | 9.79 | 0.91 (0.86–0.97) | <0.002 |
| Peak /heart rate | 16.04 | 0.71 (0.60–0.84) | <0.0001 | 4.55 | 0.75 (0.58–0.98) | <0.033 |
| Peak work rate | 24.52 | 0.97 (0.96–0.98) | <0.0001 | 5.05 | 0.98 (0.97–0.99) | <0.025 |
| Peak heart rate | 10.23 | 0.99 (0.98–0.99) | <0.001 | 5.69 | 0.98 (0.97–0.99) | <0.017 |
| HRR | 16.86 | 0.95 (0.93–0.98) | <0.0001 | 10.66 | 0.94 (0.91–0.98) | <0.001 |
| Peak SBP | 11.06 | 0.99 (0.98–0.99) | <0.001 | 7.28 | 0.98 (0.97–0.99) | <0.007 |
AT indicates anaerobic threshold; HR, hazard ratio; HRR, heart rate recovery; mPAP, mean pulmonary arterial pressure; NT‐proBNP, N‐terminal prohormone brain natriuretic peptide; OUES, oxygen uptake efficiency slope; OUESI, oxygen uptake efficiency slope index (OUES/body surface area); PETCO2@AT, end tidal partial pressure of carbon dioxide at anaerobic threshold; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SBP, systolic blood pressure; , carbon dioxide output; , minute ventilation; , oxygen consumption; WHO‐FC, World Health Organization functional class.
The specificity and sensitivity for predicting CW and mortality and the area under the receiver operator characteristic curve (AUC) for each variable are shown in Table 3. The AUC for OUESI was higher than the slope and peak for predicting both CW and mortality. The AUC for the percent‐predicted OUES was lower than OUES for predicting both CW and mortality. In addition, the AUC for the percent‐predicted peak was also slightly lower than peak for predicting CW. The optimal cutoff values of cardiac index and OUESI for predicting CW are 2.90 L·min−1·m−2 and 0.62 m−2, respectively. The optimal cutoff values of NT‐proBNP and OUESI for predicting mortality are 1105.5 pg/mL and 0.52 m−2, respectively. When these parameters were expressed dichotomously, peak , slope, and OUES all predicted CW and mortality well. OUESI was superior to peak and slope as indicated by the log‐rank score (CW: 38.46>36.41>23.16, respectively; mortality: 29.78>12.85>21.55, respectively). Patients with OUESI ≤0.52 m−2 had a worse 5‐year survival rate than patients with OUESI >0.52 m−2 (41.9% versus 89.8%, P < 0.0001). The unadjusted HR was 8.07 (95% CI 3.31–19.66), and after adjustment for age, sex, and body mass index, the HR was 7.94 (95% CI, 3.22–19.60). Patients with OUESI ≤0.62 m−2 had a worse 5‐year CW‐free rate than patients with OUESI >0.62 m−2 (17.6% versus 83.5%, P< 0.0001). The unadjusted HR was 7.29 (95% CI, 3.51–15.15), and after adjustment for age, sex, and body mass index, the HR was 7.23 (95% CI, 3.48–15.04). The Kaplan–Meier curves of OUESI are shown in Figures 4 and 5.
Table 3.
ROC Curve Analysis for Variables in Predicting Clinical Worsening and All‐Cause Mortality
| Variables | Clinical Worsening | Mortality | ||||
|---|---|---|---|---|---|---|
| AUC | Sensitivity/Specificity, % | P Value | AUC | Sensitivity/Specificity, % | P Value | |
| WHO‐FC | 0.64 (0.56–0.69) | 59/62 | <0.0004 | 0.66 (0.59–0.72) | 69/57 | <0.001 |
| NT‐proBNP | 0.69 (0.64–0.76) | 66/66 | <0.0001 | 0.71 (0.65–0.77) | 78/58 | <0.0001 |
| RAP | 0.61 (0.54–0.68) | 49/81 | <0.0045 | 0.65 (0.59–0.72) | 59/71 | <0.0077 |
| mPAP | 0.64 (0.56–0.70) | 69/54 | <0.0008 | 0.66 (0.60–0.74) | 54/79 | <0.0012 |
| Cardiac index | 0.70 (0.64–0.76) | 85/46 | <0.0001 | 0.64 (0.56–0.69) | 44/77 | <0.0185 |
| PVR | 0.70 (0.64–0.76) | 84/50 | <0.0001 | 0.66 (0.58–0.71) | 84/40 | <0.0072 |
| Peak | 0.69 (0.62–0.75) | 76/55 | <0.0001 | 0.67 (0.60–0.74) | 86/42 | <0.0003 |
| Peak | 0.71 (0.64–0.77) | 95/42 | <0.0001 | 0.68 (0.61–0.74) | 94/45 | <0.0001 |
| Peak , % predicted | 0.69 (0.62–0.75) | 44/84 | <0.0001 | 0.68 (0.61–0.74) | 50/81 | <0.0005 |
| AT | 0.61 (0.54–0.67) | 89/44 | <0.0064 | 0.58 (0.51–0.65) | 54/62 | 0.1267 |
| OUES | 0.74 (0.67–0.80) | 64/76 | <0.0001 | 0.71 (0.65–0.77) | 75/66 | <0.0001 |
| OUESI | 0.76 (0.69–0.81) | 89/50 | <0.0001 | 0.73 (0.67–0.79) | 81/66 | <0.0001 |
| OUES, % predicted | 0.72 (0.66–0.78) | 61/74 | <0.0001 | 0.70 (0.64–0.77) | 54/80 | <0.0001 |
| slope | 0.72 (0.66–0.79) | 75/60 | <0.0001 | 0.70 (0.64–0.77) | 47/86 | <0.0001 |
| PETCO2@AT | 0.72 (0.66–0.78) | 75/62 | <0.0001 | 0.69 (0.62–0.75) | 64/72 | <0.0004 |
| Peak /heart rate | 0.65 (0.58–0.71) | 68/55 | <0.0001 | 0.64 (0.57–0.70) | 78/54 | <0.0091 |
| Peak work rate | 0.68 (0.61–0.74) | 55/74 | <0.0001 | 0.60 (0.54–0.66) | 40/79 | 0.0956 |
| Peak heart rate | 0.64 (0.56–0.69) | 46/74 | <0.0009 | 0.58 (0.51–0.65) | 40/85 | 0.1879 |
| HRR | 0.64 (0.57–0.70) | 42/81 | <0.0005 | 0.66 (0.59–0.74) | 54/76 | <0.0024 |
| Peak SBP | 0.67 (0.60–0.74) | 67/64 | <0.0001 | 0.69 (0.62–0.75) | 88/51 | <0.0001 |
AT indicates anaerobic threshold; AUC, area under the receiver operating characteristic curve; HRR, heart rate recovery; mPAP, mean pulmonary arterial pressure; NT‐proBNP, N‐terminal prohormone brain natriuretic peptide; OUES, oxygen uptake efficiency slope; OUESI, oxygen uptake efficiency slope index (OUES/body surface area); PETCO2@AT, end tidal partial pressure of carbon dioxide at anaerobic threshold; PVR, pulmonary vascular resistance; RAP, right atrial pressure; ROC, receiver operator characteristic; SBP, systolic blood pressure; , carbon dioxide output; , minute ventilation; , oxygen consumption; WHO‐FC, World Health Organization functional class.
Figure 4.
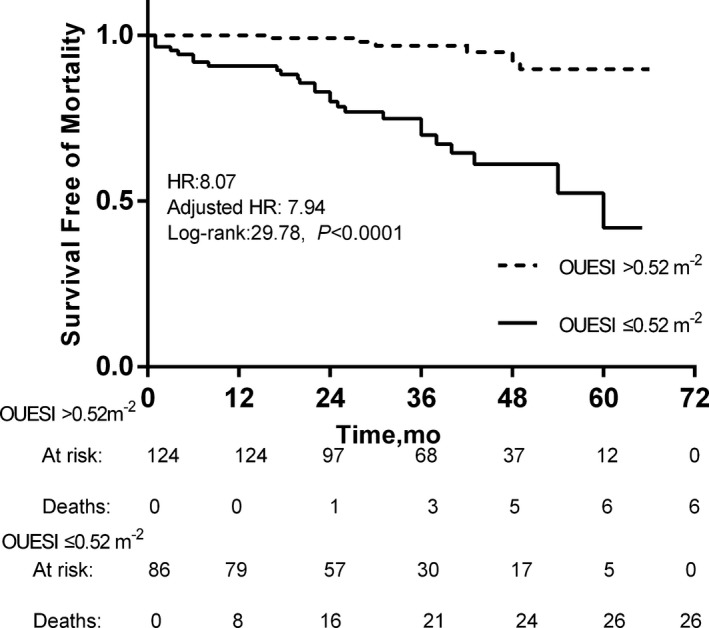
Kaplan–Meier analysis of oxygen uptake efficiency slope index (OUESI) for 5‐year mortality. Adjusted HR indicates hazard ratio (HR) adjusted for age, sex, and body mass index.
Figure 5.
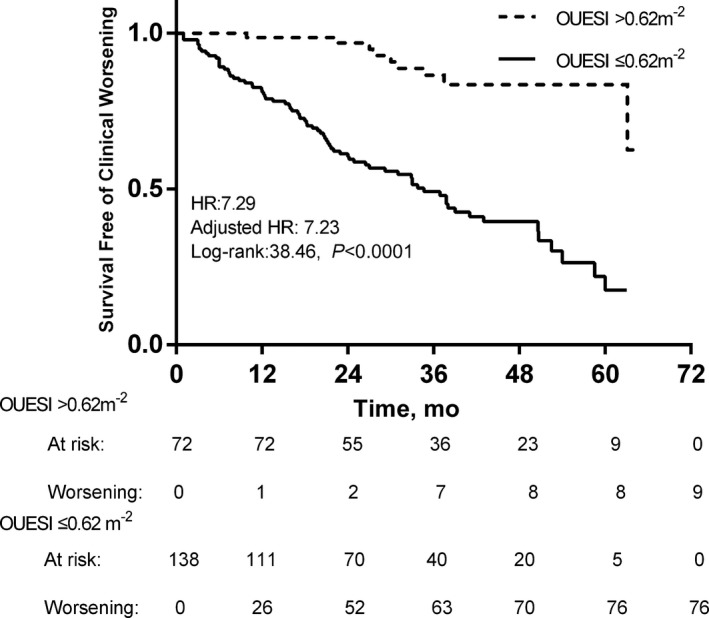
Kaplan–Meier analysis of oxygen uptake efficiency slope index (OUESI) for 5‐year clinical worsening. Adjusted HR indicates hazard ratio (HR) adjusted for age, sex, and body mass index.
Discussion
In this study, we evaluated the prognostic value of exercise parameters obtained from CPET and hemodynamic parameters from right‐sided heart catheterization and NT‐proBNP. We found that the value of OUES fell with worsening symptoms and correlated well with peak in patients with IPAH. In addition, the results of our present study found that OUESI, which was superior to peak and slope, was an independent prognostic marker of mortality and CW among exercise parameters in patients with IPAH.
OUES, a submaximal exercise parameter, represents the absolute rate of increase in oxygen consumption per 10‐fold increase in ventilation. It indicates how effectively the oxygen was extracted and utilized. Three main factors can affect OUES: (1) arterial pCO2 (CO2 set point), (2) metabolic carbon dioxide production, (3) physiologic pulmonary dead space ventilation.4, 17 Sullivan and Cobb22 demonstrated that the arterial carbon dioxide set point did not differ during exercise between patients with heart failure and normal individuals. Therefore, the distribution of blood to the skeletal muscles (systemic perfusion) determining the production of carbon dioxide and the perfusion to the lungs determining pulmonary dead space ventilation can both affect OUES.
OUES is obviously resistant to foreshortening of exercise duration, which makes it useful in the assessment of patients who are unable to perform maximal exercise.6 While peak is a maximal exercise parameter, patients with low peak can be underestimated because of lack of motivation and premature termination of exercise by the examiner. Previous studies showed that OUES was strongly correlated with peak and provided greater prognostic information over peak in patients with left‐sided heart failure.10, 23, 24 These results were consistent with the results of our study conducted in patients with IPAH.
With respect to the slope, Arena et al11 demonstrated that the slope was superior to OUES in predicting major cardiac‐related events in patients with left‐sided heart failure. However, Davies et al10 found that log OUES, rather than peak and slope, was the most powerful prognostic marker from his study of 243 patients with left‐sided heart failure and a median of 9 years of follow‐up. The proportion of β‐blocker use and the length of the tracking period may account for the difference. However, a study conducted in patients with PAH still found that OUES was the strongest independent prognostic marker, better than the slope.9 The slope relates to physiologic pulmonary dead space and indicates the status of pulmonary perfusion. OUES reflects not only the status of pulmonary perfusion but also systemic perfusion, which may be the reason why OUES provides prognostic information that is superior to that provided by the slope.9 However, patients with PAH and its associated conditions were also included in their study population, in addition to 48 patients with IPAH. In fact, Deboeck et al13 demonstrated that exercise variables obtained from a CPET were less accurate predictors of outcome in associated PAH because of inhomogeneity of the study population. Therefore, our result may be more representative when it is applied to patients with IPAH.
When OUES was corrected for BSA, it had greater power than OUES in predicting CW and mortality; however, when the predicted values for OUES were calculated according to the equations of Hollenberg and Tager, the predictive power of percent‐predicted OUES was not greater than OUES. This may have been attributable to the differences in study populations between the different studies.6, 25 The mean value of OUES in patients with IPAH in our study and other studies were obviously lower than that in patients with left‐sided heart failure (0.72–1.08 versus 1.60–1.96).7, 9, 10, 11 These results were consistent with the results from our previous studies,26, 27 which showed that patients with right‐sided heart failure had worse exercise capacity compared with patients with left‐sided heart failure.
Study Limitations
Exercise variables that predict survival in patients with IPAH cannot predict survival in patients with associated PAH13; therefore, our results only apply to patients with IPAH. Future studies need to explore whether OUES can also predict outcomes in patients with associated PAH. In addition, the sample size of our study was relatively small, and this may have led to the insignificant differences in AUC between the OUESI, slope, and peak . A multicenter, large‐scale study will be needed to validate these findings.
Conclusions
CPET provides excellent prognostic information in patients with IPAH. OUES, as an objective submaximal measure of cardiorespiratory fitness, appears to be one of the strongest prognostic markers among exercise variables for predicting CW and mortality.
Sources of Funding
This work was supported by the Central Public Interest Scientific Institution Basal Research Fund for Young Researchers (No. 2010F11).
Disclosures
None.
Acknowledgments
We thank Xinguo Sun for his assistance with technical guidance.
(J Am Heart Assoc. 2017;6:e005037 DOI: 10.1161/JAHA.116.005037.)28666992
References
- 1. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk NA, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barberà J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol Ç, Falk V, Funck‐Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Völler H, Luis Zamorano J. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119.26320113 [Google Scholar]
- 2. Pinkstaff SO, Burger CD, Daugherty J, Bond S, Arena R. Cardiopulmonary exercise testing in patients with pulmonary hypertension: clinical recommendations based on a review of the evidence. Expert Rev Respir Med. 2016;10:279–295. [DOI] [PubMed] [Google Scholar]
- 3. Arena R, Lavie CJ, Milani RV, Myers J, Guazzi M. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence‐based review. J Heart Lung Transplant. 2010;29:159–173. [DOI] [PubMed] [Google Scholar]
- 4. Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28:1567–1572. [DOI] [PubMed] [Google Scholar]
- 5. Baba R. The oxygen uptake efficiency slope and its value in the assessment of cardiorespiratory functional reserve. Congest Heart Fail. 2000;6:256–258. [DOI] [PubMed] [Google Scholar]
- 6. Akkerman M, van Brussel M, Hulzebos E, Vanhees L, Helders PJ, Takken T. The oxygen uptake efficiency slope: what do we know? J Cardiopulm Rehabil Prev. 2010;30:357–373. [DOI] [PubMed] [Google Scholar]
- 7. Tan X, Yang W, Guo J, Zhang Y, Wu C, Sapkota R, Kushwaha SP, Gong S, Sun X, Liu J. Usefulness of decrease in oxygen uptake efficiency to identify gas exchange abnormality in patients with idiopathic pulmonary arterial hypertension. PLoS One. 2014;9:e98889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woods PR, Frantz RP, Taylor BJ, Olson TP, Johnson BD. The usefulness of submaximal exercise gas exchange to define pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramos RP, Ota‐Arakaki JS, Alencar MC, Ferreira EV, Nery LE, Neder JA. Exercise oxygen uptake efficiency slope independently predicts poor outcome in pulmonary arterial hypertension. Eur Respir J. 2014;43:1510–1512. [DOI] [PubMed] [Google Scholar]
- 10. Davies LC, Wensel R, Georgiadou P, Cicoira M, Coats AJ, Piepoli MF, Francis DP. Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non‐linear analysis: oxygen uptake efficiency slope. Eur Heart J. 2006;27:684–690. [DOI] [PubMed] [Google Scholar]
- 11. Arena R, Myers J, Hsu L, Peberdy MA, Pinkstaff S, Bensimhon D, Chase P, Vicenzi M, Guazzi M. The minute ventilation/carbon dioxide production slope is prognostically superior to the oxygen uptake efficiency slope. J Card Fail. 2007;13:462–469. [DOI] [PubMed] [Google Scholar]
- 12. Peacock AJ, Naeije R, Galie N, Rubin L. End‐points and clinical trial design in pulmonary arterial hypertension: have we made progress? Eur Respir J. 2009;34:231–242. [DOI] [PubMed] [Google Scholar]
- 13. Deboeck G, Scoditti C, Huez S, Vachiery JL, Lamotte M, Sharples L, Melot C, Naeije R. Exercise testing to predict outcome in idiopathic versus associated pulmonary arterial hypertension. Eur Respir J. 2012;40:1410–1419. [DOI] [PubMed] [Google Scholar]
- 14. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez‐Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G; ESC Committee for Practice Guidelines (CPG) . Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493–2537. [DOI] [PubMed] [Google Scholar]
- 15. Tabet JY, Beauvais F, Thabut G, Tartiere JM, Logeart D, Cohen‐Solal A. A critical appraisal of the prognostic value of the VE/VCO2 slope in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2003;10:267–272. [DOI] [PubMed] [Google Scholar]
- 16. Ferreira EV, Ota‐Arakaki JS, Ramos RP, Barbosa PB, Almeida M, Treptow EC, Valois FM, Nery LE, Neder JA. Optimizing the evaluation of excess exercise ventilation for prognosis assessment in pulmonary arterial hypertension. Eur J Prev Cardiol. 2014;21:1409–1419. [DOI] [PubMed] [Google Scholar]
- 17. Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol. 2000;36:194–201. [DOI] [PubMed] [Google Scholar]
- 18. Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–S55. [DOI] [PubMed] [Google Scholar]
- 19. Wasserman K, Hansen JE, Sue DY, Stringer W, Whipp BJ. Normal values In: Weinberg R, ed. Principles of Exercise Testing and Interpretation. 4th ed Philadelphia, PA: Lippincott Williams and Wilkins; 2005:160–182. [Google Scholar]
- 20. Zhang HL, Liu ZH, Wang Y, Xiong CM, Ni XH, He JG, Luo Q, Zhao ZH, Zhao Q, Sun XG. Acute responses to inhalation of iloprost in patients with pulmonary hypertension. Chin Med J (Engl). 2012;125:2826–2831. [PubMed] [Google Scholar]
- 21. Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan MJ, Cobb FR. The anaerobic threshold in chronic heart failure. Relation to blood lactate, ventilatory basis, reproducibility, and response to exercise training. Circulation. 1990;81:II47–II58. [PubMed] [Google Scholar]
- 23. Van Laethem C, Bartunek J, Goethals M, Nellens P, Andries E, Vanderheyden M. Oxygen uptake efficiency slope, a new submaximal parameter in evaluating exercise capacity in chronic heart failure patients. Am Heart J. 2005;149:175–180. [DOI] [PubMed] [Google Scholar]
- 24. Lin YS, Huang HY, Lin WH, Wei J, Chen JC, Kuo LY, Hsu CL, Chen BY, Cheng FH. Oxygen uptake efficiency slope predicts major cardiac events in patients with end‐stage heart failure. Transplant Proc. 2016;48:956–958. [DOI] [PubMed] [Google Scholar]
- 25. Marinov B, Mandadzhieva S, Kostianev S. Oxygen‐uptake efficiency slope in healthy 7‐ to 18‐year‐old children. Pediatr Exerc Sci. 2007;19:159–170. [DOI] [PubMed] [Google Scholar]
- 26. Liu WH, Luo Q, Liu ZH, Zhao Q, Xi QY, Zhao ZH. Differences in exercise capacity in patients with chronic left heart failure and chronic right heart failure. Heart Lung Circ. 2014;23:1036–1040. [DOI] [PubMed] [Google Scholar]
- 27. Liu WH, Luo Q, Liu ZH, Zhao Q, Xi QY, Zhao ZH. Differences in pulmonary function and exercise capacity in patients with idiopathic dilated cardiomyopathy and idiopathic pulmonary arterial hypertension. Heart Lung. 2014;43:317–321. [DOI] [PubMed] [Google Scholar]


