Abstract
Background
Lower socioeconomic position (SEP) predicts higher cardiovascular risk in adults. Few studies differentiate between neighborhood and family SEP or have repeated measures through childhood, which would inform understanding of potential mechanisms and the timing of interventions. We investigated whether neighborhood and family SEP, measured biennially from ages 0 to 1 year onward, was associated with carotid intima–media thickness (IMT) at ages 11 to 12 years.
Methods and Results
Data were obtained from 1477 families participating in the Child Health CheckPoint study, nested within the Longitudinal Study of Australian Children. Disadvantaged family and neighborhood SEP was cross‐sectionally associated with thicker maximum carotid IMT in separate univariable linear regression models. Associations with family SEP were not attenuated in multivariable analyses, and associations with neighborhood SEP were attenuated only in models adjusted for family SEP. The difference in maximum carotid IMT between the highest and lowest family SEP quartile measured at ages 10 to 11 years was 10.7 μm (95% CI, 3.4–18.0; P=0.004), adjusted for age, sex, pubertal status, passive smoking exposure, body mass index, blood pressure, and arterial lumen diameter. In longitudinal analyses, family SEP measured as early as age 2 to 3 years was associated with maximum carotid IMT at ages 11 to 12 years (difference between highest and lowest quartile: 8.5 μm; 95% CI, 1.3–15.8; P=0.02). No associations were observed between SEP and mean carotid IMT.
Conclusions
We report a robust association between lower SEP in early childhood and carotid IMT in mid‐childhood. Further investigation of mechanisms may inform pediatric cardiovascular risk assessment and prevention strategies.
Keywords: atherosclerosis, CheckPoint, prevention, socioeconomic position, subclinical atherosclerosis risk factor
Subject Categories: Cardiovascular Disease, Pediatrics, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
We present the first evidence that socioeconomic position, no matter when measured across the first decade of life, is associated with carotid intima–media thickness at ages 11 to 12 years.
Children in the lowest socioeconomic quartile at 11 to 12 years of age were 46% more likely to have a carotid intima–media thickness >75th percentile—this corresponds to a vascular age >8 years older than their 50th‐percentile counterparts.
The association is independent of age, sex, puberty, and traditional risk factors such as body mass index, blood pressure, and passive smoking exposure.
What Are the Clinical Implications?
Family, rather than neighborhood, socioeconomic position in the first 10 years of life may be an important indicator of early subclinical atherosclerosis.
The long‐term implications of increased intima–media thickness at ages 11 to 12 years need to be confirmed.
Carotid ultrasound may be a useful component of pediatric cardiovascular risk assessment.
Introduction
The association between lower socioeconomic position (SEP) and higher risk of cardiovascular disease (CVD) mortality and morbidity is an enduring relationship in cardiovascular epidemiology.1, 2 Understanding when associations between SEP and CVD first appear may help address the increasing social gradients in CVD outcomes and risk factors.1, 3 Preventative strategies in childhood are more effective than those initiated in adulthood, as childhood is when important health behaviors such as diet and physical activity patterns are established.4 Reducing social disparities in CVD requires better understanding of the early life determinants of atherosclerosis.
Carotid intima–media thickness (IMT) allows noninvasive quantification of the early development of atherosclerosis.5 It is an important predictor of later life cardiovascular events in adults6, 7 and reflects the burden of atherosclerosis at autopsy.8 Traditional risk factors such as hyperlipidemia and hypertension are cross‐sectionally associated with thicker carotid IMT in both adults and children,5 and childhood risk factors predict adult carotid IMT in longitudinal studies.9
Lower SEP is cross‐sectionally associated with thicker carotid IMT in adults,10, 11, 12, 13 and evidence suggests that this association may already be apparent in adolescence.14 Most studies in younger people are limited by modest sample sizes with wide age ranges, leading to potential confounding, for example, by pubertal stage. Moreover, the distinction between family SEP and neighborhood SEP is often overlooked,15 and few studies have assessed both measures longitudinally in the same study.
To address these knowledge gaps, we analyzed data from the Child Heath CheckPoint (CheckPoint), nested within the Longitudinal Study of Australian Children (LSAC), to investigate whether and when in the life course lower family or neighborhood SEP measures were associated with thicker carotid IMT at ages 11 to 12 years.
Methods
Study Design and Participants
In LSAC, 5107 healthy infants were randomly recruited in 2004 at 0 to 1 year of age as a nationally representative birth cohort. Children were followed up biennially in “waves” of data collection, collecting anthropometric, cognitive, and detailed sociodemographic questionnaire data. The response rate to the initial mailed invitation in 2004 was 57.2%, of which 73.7% (n=3764) were retained for LSAC wave 6 in 2014 (at ages 10–11 years). All contactable and consenting LSAC birth cohort families were then invited to participate in CheckPoint (n=3513), a cross‐sectional biophysical markers wave nested between LSAC waves 6 and 7. Overall, 1875 families participated in CheckPoint throughout Australia, and 1874 families gave informed consent for use of their collected data.
Ethics and inclusion criteria
CheckPoint data collection occurred in 2015 immediately after LSAC wave 6, when the children were age 11 to 12 years, and was approved by the Royal Children's Hospital (Melbourne, Australia) human research ethics committee (33225D) and the Australian Institute of Family Studies ethics committee (14‐26). Participating families were included in the current analyses if SEP data from LSAC wave 6 and carotid IMT data from CheckPoint were available (Figure 1).
Figure 1.
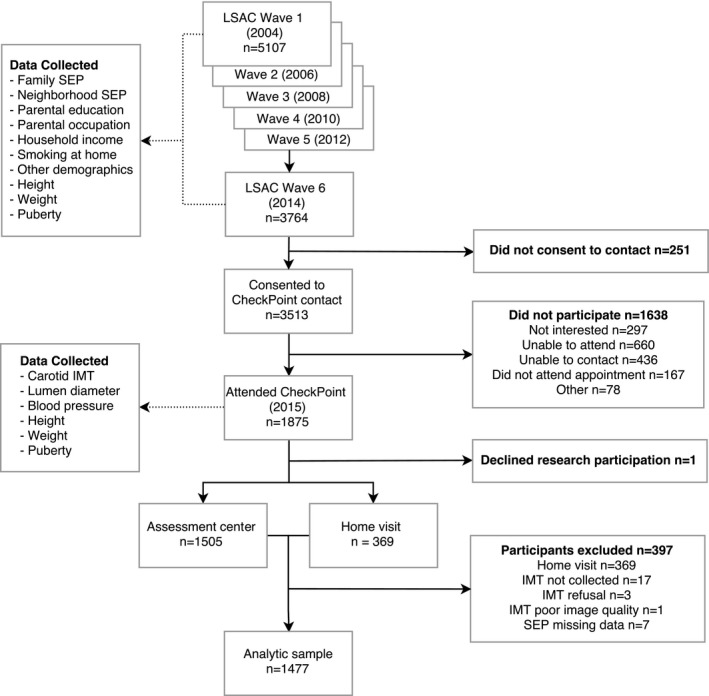
Flow diagram of participant pathway and sources of data used in analysis. CheckPoint indicates Child Health CheckPoint; IMT, intima–media thickness; LSAC, Longitudinal Study of Australian Children; SEP, socioeconomic position.
Procedures
Assessors collected data on residential postcode, parental income, education and occupation, height, weight, and puberty through parent and child questionnaires in biennial home visits from 2004 (wave 1) to 2014 (wave 6). Carotid IMT, lumen diameter, height, weight, and puberty status were also collected in 2015 at a specialized CheckPoint assessment center visit. Those families who could not arrange a visit were given the option of a home visit, at which some measures such as carotid IMT were not obtained.
Socioeconomic Position
Both neighborhood SEP and family SEP were measured in LSAC wave 6, ≈12 months before measurement of biophysical measures in CheckPoint (range: −4 to 20 months). In addition, we included longitudinal SEP measures from preceding LSAC waves 1 to 5 (ages 0–9 years).
Neighborhood SEP
A Socio‐Economic Indexes for Areas (SEIFA) score of the postcode region where the participating family lived was used as a measure of neighborhood SEP. The SEIFA Index of Relative Socioeconomic Advantage and Disadvantage score was a standardized score by geographic area compiled from census data to numerically summarize the social and economic conditions of Australian neighborhoods. It had a national mean of 1000 and a standard deviation of 100, where higher values represent more advantage and less disadvantage.16
Family SEP
We used a previously constructed composite measure for family SEP analyses.17 This SEP variable summarized parent‐reported combined household income, current or most recent occupation of each parent, and highest achieved educational qualification of each parent. Each component of the score was scaled, and an unweighted average was calculated over 3 values in a single‐parent household or over 5 values in a dual‐parent household. The unweighted average variable at each LSAC wave was then standardized within the wave to have a mean of 0 and SD of 1. Our main analyses used LSAC wave 6 (ages 10–11 years) family SEP.
Parental education
We defined parental education as the highest education achieved by either parent, based on the LSAC wave 6 parental questionnaires. Parental education was categorized into 5 groups a priori according to classifications previously used in LSAC analyses18: tertiary educated, completed high school (year 12) and a certificate/diploma, completed year 12 only, completed less than year 12 and a certificate/diploma, and completed less than year 12 only. For sensitivity analyses, we also collapsed the categories into those who completed tertiary education, completed high school only, and completed neither.
Parental occupational status
We defined parental occupation as the most skillful occupation held by either parent, coded according to Australian and New Zealand Standard Classification of Occupations (ANZSCO).19 Using data from LSAC wave 6 parental questionnaires, we categorized a priori highest parental occupation into 4 main categories, dependent on the occupation's required skill level. The categories included (1) manager or professional; (2) technician, tradesperson, community or personal service staff; (3) clerical or sales staff; and (4) machinery operator, driver, or laborer. We also matched ANZSCO 2‐digit codes to the Australian Socioeconomic Index 2006 (AUSEI06) occupational scores for sensitivity analyses.20 This provided a continuous numerical measure of occupation‐based SEP as an alternative way of arranging the occupations in order of skill requirement in finer detail.
Household income
Singly imputed self‐reported weekly income of each parent and other sources, derived from LSAC data, was used as a measure of combined household income.21 In the technical papers outlining the imputation process, the modified variables were considered more representative of the overall sample and more appropriate for analysis; their routine use over complete case analysis was recommended.21 We subsequently divided the combined household income by the square root of the number of family members in the household to obtain an equivalized household income that accounted for family size.
Carotid IMT
Carotid artery images were acquired using standardized protocols developed in accordance with recommendations of the American Society of Echocardiography and Mannheim Consensus statements.22, 23 All participants lay supine with their head turned 45° to the left to expose the right side of neck. The right carotid artery was chosen to harmonize with other vascular measures taken in the same assessment, such as pulse wave velocity, which also assessed the right‐sided circulation. Ultrasound images were obtained using a 10‐MHz linear array probe (Vivid I; GE Healthcare). Real‐time B‐mode ultrasound cine loops were captured in triplicate by 1 of 4 trained technicians. Modified 3‐lead ECG captured cardiac‐cycle information concurrently.
Images were transferred to digital storage and assessed by 1 technician, who selected the single best cine loop of 5 to 6 cardiac cycles. These loops were further processed using Carotid Analyzer (Medical Imaging Applications), a commercially available semiautomatic edge‐detection software program. IMT was measured ≈10 mm proximal to the carotid bulb, over a distance of 5 to 10 mm.
Mean, maximum, and higher risk IMT
Carotid IMT values are presented as the mean of 3 to 5 still frames of IMT captured at end‐diastole. We used both mean carotid IMT measurements, which referred to the average from 3‐ to 5‐ frames of the entire carotid IMT measurement over the 5‐ to 10‐mm section, and maximum carotid IMT, which referred to the 3‐ to 5‐frame mean of the thickest carotid IMT measurement over the 5‐ to 10‐mm section.
Six trained raters measured all cine loops. Training consisted of 30 example cine loops that were subsequently assessed for consistency by an expert rater (R.S.L.). We assessed protocol drift and reliability between raters by randomly reanalyzing a subset of 105 images in quadruplicate. Inter‐ and intrarater reliability of the carotid IMT measurement was good and comparable to other published results.24 The within‐observer coefficients of variation were 6.5% and 4.9% for mean and maximum carotid IMT values, respectively, and the between‐observer coefficients of variation were 9.5% and 6.2%, respectively. Within‐observer intraclass correlations were 0.71 (95% confidence interval [CI], 0.63–0.78) and 0.62 (95% CI, 0.54–0.71), respectively. Between‐observer intraclass correlations were 0.64 (95% CI, 0.54–0.74) and 0.59 (95% CI, 0.49–0.68).
To provide clinical context for our findings, carotid IMT measurements >75th percentile in our sample were categorized as higher risk carotid IMT for both mean and maximum carotid IMT measurements.
Other Sample Characteristics
Age, sex, and puberty
Age at assessment was collected at each wave by calculating the days between the participant's date of birth and date of assessment. Sex and pubertal stage were determined by self‐report in questionnaires. Participants were categorized into prepubertal, early pubertal, midpubertal, late pubertal, and postpubertal stages using the Pubertal Development Scale.25
Anthropometry
Using a portable stadiometer, participant height was measured without shoes, in light clothing, and in duplicate, to the nearest 0.1 cm. A third measurement was taken if the difference of the first 2 height measurements was >0.5 cm; final height was the mean of all measurements made. Weight, to the nearest 0.1 kg, was measured with an InBody230 bioelectrical impedance analysis scale. An age‐ and sex‐adjusted body mass index z score was calculated using the US Centers for Disease Control and Prevention growth reference charts.26
Blood pressure
Blood pressure was measured using a SphygmoCor XCEL system (AtCor Medical Pty Ltd). Preceded by 7 minutes in supine position at rest, systolic and diastolic blood pressures were measured at the brachial artery up to 3 times. The mean values for systolic and diastolic blood pressure were used (shown as SBP and DBP, respectively) in the following equation to derive an estimated mean arterial pressure (shown as MAP):
Smoking at home and other demographics
In a written questionnaire at each LSAC wave, parents reported the number of smokers at home. We created a binary variable of “ever exposed to passive smoke” from these data; it was positive for the child if the answer to the smokers‐at‐home question in any LSAC wave (ages 0–11 years) was >0. Details of Aboriginal or Torres Strait Islander status and whether other languages were spoken at home were collected from parent report at wave 1 and used as binary variables in analysis.
Statistical Analyses
All statistical analyses were performed using Stata 14.1 (StataCorp LP). Quartiles were based on the analytic sample (n=1477). We addressed the first aim of our study by constructing linear regression models using carotid IMT as the outcome. Both neighborhood SEP and family SEP from LSAC wave 6 were used as categorical variables split at sample derived quartiles. The exposure variable was modeled both linearly, assuming each quartile increment would have the same magnitude of effect, and categorically in comparison to the baseline lowest quartile group. Covariates were selected a priori to be included in adjusted models. Additional adjustment for both neighborhood and family SEP in the same model was performed to examine which variable explained more of the variance in carotid IMT.
We subsequently explored the components of family SEP using categorical SEP variables in linear regression models. Covariates were selected a priori to be included in adjusted models, and adjustment for each SEP component was undertaken in the further adjusted model.
We used modified Poisson regression models27 to estimate the relative risk of having a higher risk carotid IMT between exposure quartiles of family and neighborhood SEP and components of family SEP.
Equivalent neighborhood and family SEP variables collected in previous study waves were used as categorical variables in analyses replicating the first aim to address whether time of measurement of SEP was relevant. Each model included the carotid IMT outcome at CheckPoint, with SEP exposure at the single time point of each previous wave.
We used multiple imputation with the method of chained equations28 to generate plausible values for missing data to recover information from incomplete cases and to reduce potential biases introduced by complete case analysis. Imputation was conducted for all variables with missing data (Table 1), and results were compared with complete case analysis. The imputation model included all analytic variables as well as Aboriginal and Torres Strait Islander status and whether a language other than English was spoken at home. Twenty data sets were imputed, and analyses were performed using the mi set of commands in Stata. Results with and without imputation were comparable; we chose to present complete case analyses as our main results and included any notable differences from imputation in our description of these sensitivity analyses.
Table 1.
Number of Participants in Analytic Sample (n=1477) With Missing Data for Variables Used in Analyses
| Characteristics | Participants With Missing Data, n (%) |
|---|---|
| Family SEP | 0 (0.0) |
| Neighborhood SEP | 0 (0.0) |
| Male sex | 0 (0.0) |
| Age | 0 (0.0) |
| Aboriginal or Torres Strait Islander | 0 (0.0) |
| Non‐English language at home | 0 (0.0) |
| Ever exposed to smoke at home | 1 (0.1) |
| Height | 1 (0.1) |
| Weight | 1 (0.1) |
| Body mass index z score, CDC | 1 (0.1) |
| Systolic blood pressure | 100 (6.8) |
| Diastolic blood pressure | 100 (6.8) |
| Estimated mean arterial pressure | 100 (6.8) |
| Puberty development category | 111 (7.5) |
| Highest parental education category | 0 (0.0) |
| Highest parental occupational category | 18 (1.2) |
| Equivalized household income | 62 (4.2) |
| Mean carotid IMT | 0 (0.0) |
| Maximum carotid IMT | 0 (0.0) |
| Minimum lumen–lumen diameter | 69 (4.7) |
CDC indicates Centers for Disease Control and Prevention; IMT, intima–media thickness; SEP, socioeconomic position.
Results
Characteristics of the Study Participants
We collected SEP data and child carotid IMT measurements from 1477 families (79% of the total CheckPoint cohort; Figure 1). In attrition analyses, the present cohort had higher family and neighborhood SEP compared with those who did not undertake carotid IMT measurements in the latest follow‐up and those who did not participate in the follow‐up (Table 2).29 The sample mean family SEP was 0.24 SD above the mean SEP of all families at LSAC wave 6 and 0.37 SD above the mean SEP of all 5107 families enrolled in LSAC. The Pearson correlation coefficient between family and neighborhood SEP at LSAC wave 6 was 0.37.
Table 2.
Characteristics of Excluded Participants
| Characteristics | CheckPoint (Age 11–12 Y) | Wave 6 (Age 10–11 Y) | Wave 1 (Age 0–1 Y) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Analytic Sample (n=1477) | Excluded (n=397) | Total (n=1874) | Analytic Sample (n=1477) | Excluded (n=2287) | Total (n=3764) | Analytic Sample (n=1477) | Excluded (n=3630) | Total (n=5107) | |
| Family SEP at assessment | 0.24 (0.97) | −0.09 (1.02) | 0.17 (0.99) | 0.24 (0.97) | −0.16 (0.99) | 0.00 (1.00) | 0.37 (0.94) | −0.15 (0.98) | 0.00 (1.00) |
| Neighborhood SEP at assessment | 1032 (68) | 1013 (70) | 1028 (69) | 1032 (68) | 1005 (74) | 1016 (73) | 1021 (83) | 997 (75) | 1004 (79) |
| Male sex (%) | 738 (50.0) | 217 (54.7) | 955 (51.0) | 738 (50.0) | 1191 (52.1) | 1929 (51.2) | 738 (50.0) | 1870 (51.5) | 2608 (51.1) |
| Age at assessment, y | 11.9 (0.4) | 12.2 (0.4) | 11.9 (0.4) | 10.9 (0.3) | 10.9 (0.3) | 10.9 (0.3) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) |
| Aboriginal or Torres Strait Islander (%) | 21 (1.4) | 16 (4.0) | 37 (2.0) | 21 (1.4) | 85 (3.7) | 106 (2.8) | 21 (1.4) | 209 (5.8) | 230 (4.5) |
| Non‐English language at home (%) | 104 (7.0) | 44 (11.1) | 148 (7.9) | 104 (7.0) | 225 (9.8) | 329 (8.7) | 104 (7.0) | 437 (12.0) | 541 (10.6) |
| Ever exposed to smoke at home (%) | 211 (14.3) | 88 (22.2) | 299 (16.0) | 211 (14.3) | 588 (25.7) | 799 (21.2) | 106 (7.7) | 541 (18.2) | 647 (14.9) |
| Height at assessment, cm | 153.5 (8.0) | 155.4 (7.9) | 153.9 (8.0) | 146.3 (7.1) | 146.2 (7.2) | 146.2 (7.1) | 0.3 (1.2)a | 0.2 (1.2)a | 0.2 (1.2)a |
| Weight at assessment, kg | 45.4 (10.4) | 47.5 (11.3) | 45.8 (10.6) | 39.9 (8.6) | 40.9 (10.1) | 40.5 (9.6) | 0.0 (1.1)a | −0.1 (1.1)a | 0.0 (1.1)a |
| Body mass index z score, CDC | 0.30 (0.97) | 0.32 (1.05) | 0.31 (0.99) | 0.22 (1.10) | 0.31 (1.24) | 0.27 (1.19) | ··· | ··· | ··· |
| Systolic blood pressure, mm Hg | 108.2 (8.0) | 108.0 (8.6) | 108.1 (8.1) | 97.0 (10.6) | 98.1 (11.4) | 97.6 (11.1) | ··· | ··· | ··· |
| Diastolic blood pressure, mm Hg | 62.9 (5.6) | 60.7 (6.4) | 62.41 (5.9) | 58.2 (8.0) | 58.9 (8.6) | 58.6 (8.3) | ··· | ··· | ··· |
| Estimated mean arterial pressure, mm Hg | 78.0 (5.7) | 76.5 (6.4) | 77.66 (5.9) | 71.1 (7.7) | 72.0 (8.5) | 71.6 (8.2) | ··· | ··· | ··· |
| Puberty development category (%) | ··· | ··· | ··· | ||||||
| Prepubertal | 138 (10.1) | 27 (7.4) | 165 (9.5) | 606 (41.3) | 851 (38.9) | 1457 (39.9) | ··· | ··· | ··· |
| Early pubertal | 346 (25.3) | 99 (27.1) | 445 (25.7) | 385 (26.2) | 626 (28.6) | 1011 (27.7) | ··· | ··· | ··· |
| Midpubertal | 713 (52.2) | 170 (46.6) | 883 (51.0) | 440 (30.0) | 627 (28.7) | 1067 (29.2) | ··· | ··· | ··· |
| Late pubertal | 165 (12.1) | 64 (17.5) | 229 (13.2) | 36 (2.5) | 80 (3.7) | 116 (3.2) | ··· | ··· | ··· |
| Postpubertal | 4 (0.3) | 5 (1.4) | 9 (0.5) | 0 (0.0) | 4 (0.2) | 4 (0.1) | ··· | ··· | ··· |
| Highest parental education category at assessment (%) | |||||||||
| Tertiary graduate | 874 (59.2) | 176 (44.3) | 1050 (56.0) | 874 (59.2) | 952 (41.8) | 1826 (48.6) | 822 (56.7) | 1148 (36.4) | 1970 (42.8) |
| Y 12 and diploma/certificate | 346 (23.4) | 113 (28.5) | 459 (24.5) | 346 (23.4) | 633 (27.8) | 979 (26.1) | 328 (22.6) | 853 (27.0) | 1181 (25.7) |
| Y 12 only | 82 (5.6) | 43 (10.8) | 125 (6.7) | 82 (5.6) | 200 (8.8) | 282 (7.5) | 129 (8.9) | 374 (11.9) | 503 (10.9) |
| Y <12 and diploma/certificate | 149 (10.1) | 51 (12.8) | 200 (10.7) | 149 (10.1) | 392 (17.2) | 541 (14.4) | 136 (9.4) | 519 (16.5) | 655 (14.2) |
| Y <12 only | 26 (1.8) | 14 (3.5) | 40 (2.1) | 26 (1.8) | 102 (4.5) | 128 (3.4) | 34 (2.3) | 261 (8.3) | 295 (6.4) |
| Highest parental occupational category at assessment (%) | |||||||||
| Manager/professional (ANZSCO 1–2) | 979 (67.1) | 218 (56.5) | 1197 (64.9) | 979 (67.1) | 1177 (53.7) | 2156 (59.0) | 856 (61.5)b | 1346 (48.3)b | 2202 (52.7)b |
| Trade/services (ANZSCO 3–4) | 283 (19.4) | 98 (25.4) | 381 (20.7) | 283 (19.4) | 565 (25.8) | 848 (23.2) | 295 (21.2)b | 738 (26.5)b | 1033 (24.7)b |
| Clerical/sales (ANZSCO 5–6) | 152 (10.4) | 53 (13.7) | 205 (11.1) | 152 (10.4) | 294 (13.4) | 446 (12.2) | 159 (11.4)b | 384 (13.8)b | 543 (13.0)b |
| Laborer/driver/operator (ANZSCO 7–8) | 45 (3.1) | 17 (4.4) | 62 (3.4) | 45 (3.1) | 157 (7.2) | 202 (5.5) | 82 (5.9)b | 318 (11.4)b | 400 (9.6)b |
| Equivalized household income at assessment, $AUD/week | 1140 (794–1579) | 995 (625–1379) | 1100 (751–1547) | 1140 (794–1579) | 957 (608–1379) | 1027 (670–1455) | 767 (553–1032) | 642 (424–894) | 674 (460–950) |
Continuous, normally distributed variables were reported as mean (standard deviation) and categorical variables as number (%). Equivalized household income (in AUD) calculated as household (parental and other) weekly income divided by the square root of the number of family members, summarized by median (interquartile range). The final CheckPoint sample size was 1874 because 1 family declined to share their data after participation. ANZSCO indicates Australian and New Zealand Standard Classification of Occupations; AUD, Australian dollars; CDC, Centers for Disease Control and Prevention; CheckPoint, Child Health CheckPoint; IMT, intima–media thickness; LSAC, Longitudinal Study of Australian Children; SEP, socioeconomic position.
Birth weight and height z scores, based on Australian sex and gestational age reference values.29
Parental occupation categories were measured at LSAC wave 2 (age 2–3 years).
The study sample trends of family SEP are outlined in Table 3. Gradients across family SEP quartiles (with a consistent increase or decrease in mean values per quartile) were observed in Aboriginal or Torres Strait Islander status, passive smoking exposure, child weight, blood pressure, and pubertal development. The mean–mean and mean–maximum carotid IMTs were 497 μm (SD 59 μm) and 581 μm (47 μm), respectively. The Pearson correlation coefficient between mean and maximum carotid IMT was 0.76.
Table 3.
Trends in Sample Characteristics by Quartile of LSAC Wave 6 Family SEP
| Characteristic | Total (n=1477) | By Family SEP Quartile (at Age 10–11 Y) | |||
|---|---|---|---|---|---|
| Highest (n=370) | Second (n=369) | Third (n=369) | Lowest (n=369) | ||
| Family SEPa | 0.24 (0.97) | 1.44 (0.35) | 0.61 (0.19) | −0.09 (0.21) | −0.99 (0.61) |
| Neighborhood SEPa | 1032 (68) | 1062 (58) | 1040 (67) | 1022 (66) | 1004 (68) |
| Male sex (%) | 738 (50.0) | 183 (49.5) | 184 (49.9) | 172 (46.6) | 199 (53.9) |
| Age at assessment, y | 11.9 (0.4) | 11.9 (0.4) | 11.8 (0.4) | 11.9 (0.4) | 11.9 (0.4) |
| Aboriginal or Torres Strait Islanderb (%) | 21 (1.4) | 2 (0.5) | 1 (0.3) | 8 (2.2) | 10 (2.7) |
| Non‐English language at homeb (%) | 104 (7.0) | 20 (5.4) | 29 (7.9) | 29 (7.9) | 26 (7.0) |
| Ever exposed to smoke at homeb (%) | 211 (14.3) | 18 (4.9) | 34 (9.2) | 64 (17.3) | 95 (25.8) |
| Child height, cm | 153.5 (8.0) | 153.5 (8.3) | 153.2 (7.8) | 153.8 (7.5) | 153.4 (8.3) |
| Child weight, kg | 45.4 (10.4) | 44.1 (9.0) | 44.8 (9.8) | 45.8 (10.9) | 46.9 (11.4) |
| Body mass index z score, CDC | 0.30 (0.97) | 0.17 (0.88) | 0.28 (0.94) | 0.30 (1.03) | 0.46 (1.01) |
| Mean systolic blood pressure, mm Hg | 108.2 (8.0) | 107.2 (7.4) | 107.8 (8.1) | 108.8 (7.9) | 109.0 (8.4) |
| Mean diastolic blood pressure, mm Hg | 62.9 (5.6) | 62.3 (5.5) | 62.5 (5.7) | 63.2 (5.2) | 63.4 (6.1) |
| Estimated mean arterial blood pressure, mm Hg | 78.0 (5.7) | 77.3 (5.4) | 77.6 (5.8) | 78.4 (5.4) | 78.6 (6.2) |
| Pubertal development category (%) | |||||
| Prepubertal | 138 (10.1) | 37 (10.7) | 36 (10.5) | 41 (12.1) | 24 (7.1) |
| Early pubertal | 346 (25.3) | 107 (30.8) | 81 (23.6) | 76 (22.4) | 82 (24.4) |
| Midpubertal | 713 (52.2) | 171 (49.3) | 181 (52.8) | 183 (53.8) | 178 (53.0) |
| Late pubertal | 165 (12.1) | 31 (8.9) | 45 (13.1) | 40 (11.8) | 49 (14.6) |
| Postpubertal | 4 (0.3) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 3 (0.9) |
| Highest parental education categorya (%) | |||||
| Tertiary graduate | 874 (59.2) | 369 (99.7) | 327 (88.6) | 149 (40.4) | 29 (7.9) |
| Y 12 and diploma/certificate | 346 (23.4) | 1 (0.3) | 35 (9.5) | 165 (44.7) | 145 (39.3) |
| Y 12 only | 82 (5.6) | 0 (0.0) | 3 (0.8) | 20 (5.4) | 59 (16.0) |
| Y <12 and diploma/certificate | 149 (10.1) | 0 (0.0) | 4 (1.1) | 34 (9.2) | 111 (30.1) |
| Y <12 only | 26 (1.8) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 25 (6.8) |
| Highest parental occupational categorya (%) | |||||
| Manager/professional (ANZSCO 1–2) | 979 (67.1) | 370 (100.0) | 329 (89.2) | 223 (60.4) | 57 (16.2) |
| Trade/services (ANZSCO 3–4) | 283 (19.4) | 0 (0.0) | 27 (7.3) | 92 (24.9) | 164 (46.7) |
| Clerical/sales (ANZSCO 5–6) | 152 (10.4) | 0 (0.0) | 13 (3.5) | 53 (14.4) | 86 (24.5) |
| Laborer/driver/operator (ANZSCO 7–8) | 45 (3.1) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 44 (12.5) |
| Equivalized household incomea, $AUD/week | 1140 (794–1579) | 1703 (1296–2392) | 1253 (957–1646) | 1027 (766–1286) | 716 (433–995) |
| Far wall intima–media thickness | |||||
| Mean, μm | 497.1 (58.9) | 494.5 (58.1) | 499.1 (60.1) | 495.7 (60.9) | 499.0 (56.6) |
| Maximum, μm | 581.3 (46.7) | 575.9 (41.7) | 579.3 (49.7) | 582.8 (49.0) | 587.1 (45.3) |
| Minimum lumen diameter, mm | 4.85 (0.41) | 4.90 (0.38) | 4.84 (0.42) | 4.83 (0.42) | 4.85 (0.42) |
Continuous, normally distributed variables were reported as mean (standard deviation), and categorical variables as number (%). Equivalized household income ($AUD) calculated as household (parental and other) weekly income divided by the square root of the number of family members, summarized by median (IQR). One‐digit codes of parental occupation grouped into 4 categories according to ANZSCO category skill level from most to least skilled: Managers (1), Professionals (2), Technicians and Trade Workers (3), Community and Personal Service Workers (4), Clerical and Administrative Workers (5), Sales Workers (6), Machinery Operators and Drivers (7), Laborers (8). All measures derived from CheckPoint (age 11–12 years) unless otherwise indicated. ANZSCO indicates Australian and New Zealand Standard Classification of Occupations; AUD, Australian dollars; CDC, Centers for Disease Control and Prevention; LSAC, Longitudinal Study of Australian Children; SEP, socioeconomic position.
Measured at LSAC wave 6 (age 10–11).
Summary of all LSAC waves (age 0–11).
Family and Neighborhood SEP
There was evidence of a cross‐sectional association between both family and neighborhood SEP with maximum carotid IMT but not with mean carotid IMT (Table 4). When SEP was treated as a categorical variable, the difference in maximum carotid IMT between the highest and lowest quartiles of family SEP measured at ages 10 to 11 years was 10.7 μm (95% CI, 3.4–18.0; P=0.004) in linear regression models adjusted for age, sex, body mass index z score, mean arterial blood pressure, pubertal category, minimal intima–intima lumen diameter, and exposure to passive smoking. In Table 4 (and for P‐value calculations in Figure 2), SEP was used as a quasicontinuous variable with results presented per unit increment of SEP quartile to observe linear trends across the whole sample. In univariable analyses, each quartile increment higher of family SEP was associated with a 3.7‐μm thicker carotid IMT (95% CI, 1.6–5.8; P=0.001); the equivalent neighborhood SEP quartile increment was associated with a 2.6‐μm thicker carotid IMT (95% CI, 0.4–4.8; P=0.02). In multivariable analysis including both SEP variables and previously mentioned covariates, the association between family SEP and maximum carotid IMT remained statistically significant (model 3, β=3.0; 95% CI, 0.6–5.4; P=0.02), whereas the neighborhood SEP association attenuated toward the null hypothesis (β=1.2; 95% CI, −1.3 to 3.6; P=0.34).
Table 4.
Unadjusted and Adjusted Linear Regression Models of Mean and Maximum Carotid IMT and Family and Neighborhood SEP
| LSAC Wave 6 SEP Measures, at Age 10–11 Y | Mean Carotid IMT | Maximum Carotid IMT | ||
|---|---|---|---|---|
| Regression Coefficient, μm (95% CI) | P Value | Regression Coefficient, μm (95% CI) | P Value | |
| Unadjusted | ||||
| Family (per quartile lower) | 1.0 (−1.7, 3.7) | 0.46 | 3.7 (1.6, 5.8) | 0.001 |
| Neighborhood (per quartile lower) | 1.3 (−1.5, 4.1) | 0.38 | 2.6 (0.4, 4.8) | 0.02 |
| Model 1—age‐ and sex‐adjusted | ||||
| Family (per quartile lower) | 1.1 (−1.6, 3.8) | 0.41 | 3.8 (1.6, 5.9) | 0.001 |
| Neighborhood (per quartile lower) | 1.2 (−1.6, 4.0) | 0.40 | 2.6 (0.3, 4.8) | 0.02 |
| Model 2—additional covariates | ||||
| Family (per quartile lower) | 0.9 (−2.1, 3.9) | 0.56 | 3.3 (1.0, 5.7) | 0.005 |
| Neighborhood (per quartile lower) | 0.5 (−2.5, 3.5) | 0.74 | 2.1 (−0.3, 4.4) | 0.08 |
| Model 3—single model | ||||
| Family (per quartile lower) | 0.8 (−2.3, 3.9) | 0.61 | 3.0 (0.6, 5.4) | 0.02 |
| Neighborhood (per quartile lower) | 0.3 (−2.9, 3.4) | 0.87 | 1.2 (−1.3, 3.6) | 0.34 |
Regression coefficients are expressed as the μm difference in carotid IMT per quartile lower in SEP measure. Model 1 models were adjusted for age and sex, Model 2 for age, sex, body mass index z score, mean arterial blood pressure, pubertal category, minimal intima‐intima lumen diameter and exposure to passive smoking. The final Model 3 included both family and neighborhood SEP in the same model, along with all other covariates used in Model 2. Family SEP is a composite measure, standardized within each LSAC wave, summarizing parental education, occupation and household income. Neighborhood SEP uses Australian census‐derived data to generate a Socioeconomic Indexes For Areas (SEIFA) advantage/disadvantage score, according to family residential address postcode. CI indicates confidence interval; IMT, intima‐media thickness, LSAC, Longitudinal Study of Australian Children; SEP, socioeconomic position.
Figure 2.
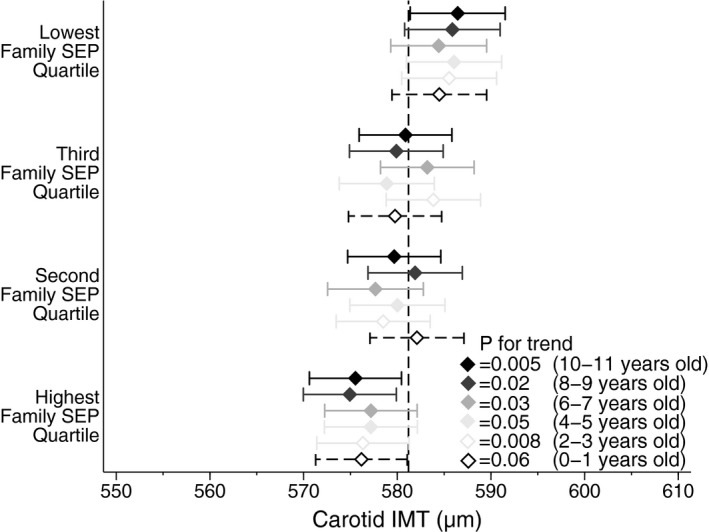
Longitudinal associations between early life family SEP (age in parentheses) and mid‐childhood maximum carotid IMT (age 11–12 years). Diamonds are adjusted point estimates of maximum carotid IMT mean according to SEP quartile. Horizontal bars indicate 95% confidence intervals of estimates. The vertical dotted line is the total sample mean. P value for each wave is for trend across quartiles. Family SEP is measured at each LSAC wave 1 to 6 (diamond color) and derived from the same parental questions on income, occupation, and education. LSAC wave 1 data used an earlier version of the occupational categorization (dashed bars) but were derived in the same way. Carotid IMT was measured after LSAC wave 6, during CheckPoint, at age 11 to 12 years. Models adjusted for age, sex, body mass index z score, mean arterial blood pressure, pubertal category, minimal intima–intima lumen diameter, and exposure to passive smoking. CheckPoint indicates Child Health CheckPoint; IMT, intima–media thickness; LSAC, Longitudinal Study of Australian Children; SEP, socioeconomic position.
In longitudinal analysis of data gathered in 2‐year intervals since ages 0 to 1 year, family SEP was consistently associated with maximum carotid IMT measured at ages 11 to 12 years. This was evident even when SEP was measured in infancy (Figure 2). When family SEP was treated as a categorical variable in a multivariable regression model, the difference in maximum carotid IMT between the highest and lowest quartiles of family SEP measured at ages 2 to 3 years was 8.5 μm (95% CI, 1.3–15.8; P=0.02). The per‐quartile increment when family SEP was modeled linearly was 3.1 μm (95% CI, 0.8–5.4; P=0.008). Similarly, consistent longitudinal patterns were also observed between neighborhood SEP and maximum carotid IMT (Figure 3).
Figure 3.
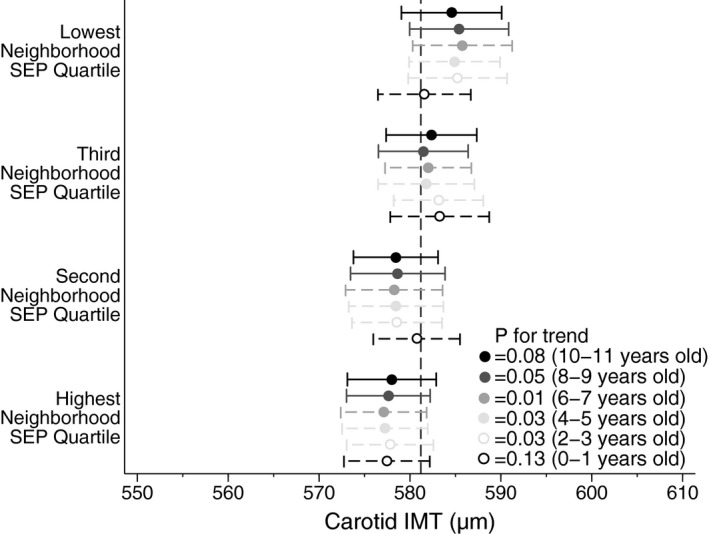
Longitudinal associations between early life neighborhood SEP (age in parentheses) and mid‐childhood maximum carotid IMT (age 11–12 years). Circles are adjusted point estimates of maximum carotid IMT mean, according to SEP quartile. Horizontal bars indicate 95% confidence intervals of estimates. The vertical dotted line is the total sample mean. P value for each wave is for trend across quartiles. Neighborhood SEP is measured using the SEIFA advantage/disadvantage score, based on child's postcode of residence (circle color) and derived from census data in 2006 (dashed horizontal bars) and 2011 (solid horizontal bars). Neighborhood SEP was measured biennially at each LSAC wave since birth (LSAC waves 1–6). Carotid IMT was measured after LSAC wave 6, during CheckPoint, at age 11 to 12 years. Models adjusted for age, sex, body mass index z score, mean arterial blood pressure, pubertal category, minimal intima–intima lumen diameter, and exposure to passive smoking. CheckPoint indicates Child Health CheckPoint; IMT, intima–media thickness; LSAC, Longitudinal Study of Australian Children; SEP, socioeconomic position.
It is important to note that correlation in SEP variables between LSAC waves was substantial. Pearson correlation coefficients between SEP measures in LSAC waves 1 to 6 ranged from 0.76 to 0.90 for family SEP and 0.56 to 0.93 for neighborhood SEP. The Pearson correlation coefficients between neighborhood and family SEP at each LSAC wave ranged from 0.32 to 0.37.
Household Income, Parental Occupation, and Parental Education
In unadjusted linear regression analyses, all 3 component variables that make up family SEP—household income, highest parental education, and highest parental occupation at child ages 10 to 11 years—were associated with the continuous measure of maximum carotid IMT at ages 11 to 12 years (Table 5). In multivariable analyses including age, sex, body mass index z score, mean arterial blood pressure, pubertal category, minimal intima–intima lumen diameter, and exposure to passive smoking as covariates, the associations between equivalized household income and highest parental occupation with maximum carotid IMT remained statistically significant (P≤0.05). None of the component SEP variables was associated with maximum carotid IMT when all components were included in the same model, suggesting substantial collinearity between different measures of family SEP. Pearson correlation coefficients were 0.46, 0.31, and 0.32 between highest parental education and occupation, between highest parental occupation and equivalized household income, and between highest parental education and equivalized household income, respectively.
Table 5.
Unadjusted and Adjusted Linear Regression Models of Mean and Maximum Carotid IMT and Components of Family SEP
| LSAC Wave 6 SEP Components, at Age 10 to 11 Y | Mean Carotid IMT | Maximum Carotid IMT | ||
|---|---|---|---|---|
| Regression Coefficient, μm (95% CI) | P Value | Regression Coefficient, μm (95% CI) | P Value | |
| Unadjusted | ||||
| Parental education category | 0.3 (−2.5, 3.1) | 0.84 | 2.5 (0.2, 4.7) | 0.03 |
| Parental occupation category | −1.3 (−5.1, 2.5) | 0.50 | 4.0 (1.0, 7.0) | 0.009 |
| Equivalized household income quartile | 1.8 (−0.9, 4.5) | 0.20 | 2.3 (0.1, 4.5) | 0.04 |
| Model 1—age‐ and sex‐adjusted | ||||
| Parental education category | 0.4 (−2.5, 3.2) | 0.81 | 2.5 (0.3, 4.7) | 0.03 |
| Parental occupation category | −1.3 (−5.1, 2.4) | 0.49 | 4.0 (1.0, 6.9) | 0.009 |
| Equivalized household income quartile | 1.7 (−1.0, 4.4) | 0.23 | 2.2 (0.1, 4.4) | 0.04 |
| Model 2—additional covariates | ||||
| Parental education category | 0.4 (−2.7, 3.6) | 0.78 | 2.3 (−0.1, 4.7) | 0.07 |
| Parental occupation category | −1.2 (−5.4, 2.9) | 0.57 | 3.6 (0.3, 6.8) | 0.03 |
| Equivalized household income quartile | 2.1 (−0.8, 5.0) | 0.16 | 2.3 (0.0, 4.6) | 0.05 |
| Model 3—single model | ||||
| Parental education category | 0.4 (−3.3, 4.2) | 0.82 | 1.6 (−1.3, 4.5) | 0.28 |
| Parental occupation category | −1.6 (−6.5, 3.3) | 0.52 | 2.5 (−1.3, 6.3) | 0.20 |
| Equivalized household income quartile | 2.4 (−0.9, 5.7) | 0.15 | 1.0 (−1.5, 3.6) | 0.43 |
Regression coefficients are expressed as the μm difference in carotid IMT per category or quartile lower in SEP measure. Model 1 models we adjusted for age and sex, Model 2 for age, sex, body mass index z score, mean arterial blood pressure, pubertal category, minimal intima‐intima lumen diameter, exposure to passive smoking, and neighborhood SEP. The final Model 3 included all components of family SEP in the same model, along with covariates used in Model 2. Family SEP is a composite measure, standardized within each LSAC Wave, summarizing parental education, occupation and household income. Neighborhood SEP uses Australian census‐derived data to generate a Socioeconomic Indexes For Areas (SEIFA) advantage/disadvantage score, according to family residential address postcode. CI indicates confidence interval; IMT, intima‐media thickness, LSAC, Longitudinal Study of Australian Children; SEP, socioeconomic position.
Carotid IMT >75th Percentile
In modified Poisson regression analyses, family SEP was inversely associated with higher risk (>75th percentile) maximum carotid IMT (Table 6). Relative risks of similar magnitude were observed with occupation category, education, and income quartiles in adjusted analyses (Table 6). The relative risk for higher risk carotid IMT in the lowest SEP quartile compared with the highest SEP quartile was 1.41 (95% CI, 1.09–1.82; P=0.008) when measured at 10 to 11 years of age and 1.43 (95% CI, 1.10–1.86; P=0.008) when measured at 2 to 3 years of age, in unadjusted models. These associations were not attenuated in multivariable models (Table 6).
Table 6.
Relative Risk for a “Higher‐Risk” Mean and Maximum Carotid IMT (>75th Percentile) at Age 11 to 12 Years
| SEP, Measured at LSAC Wave 6, at Age 10 to 11 Y | n | Carotid IMT >75th Percentile | |||
|---|---|---|---|---|---|
| Mean | Maximum | ||||
| Relative Risk (95% CI) | P Value for Trend | Relative Risk (95% CI) | P Value for Trend | ||
| Neighborhood SEP quartile | 1298 | 0.08 | 0.02 | ||
| Highest quartile | 338 | Ref | Ref | ||
| Second quartile | 316 | 1.25 (0.95, 1.65) | 1.09 (0.82, 1.45) | ||
| Third quartile | 374 | 1.40 (1.06, 1.85) | 1.30 (0.98, 1.72) | ||
| Lowest quartile | 270 | 1.25 (0.92, 1.69) | 1.35 (1.01, 1.80) | ||
| Family SEP quartile | 1298 | 0.29 | 0.01 | ||
| Highest quartile | 332 | Ref | Ref | ||
| Second quartile | 325 | 1.21 (0.92, 1.60) | 1.26 (0.94, 1.70) | ||
| Third quartile | 328 | 1.05 (0.78, 1.40) | 1.26 (0.94, 1.69) | ||
| Lowest quartile | 313 | 1.24 (0.93, 1.64) | 1.46 (1.09, 1.95) | ||
| Parental education | 1298 | 0.22 | 0.03 | ||
| Tertiary graduate | 778 | Ref | Ref | ||
| Y 12 and diploma/certificate | 303 | 0.82 (0.63, 1.07) | 0.96 (0.75, 1.23) | ||
| Y 12 only | 71 | 0.99 (0.64, 1.54) | 1.27 (0.85, 1.90) | ||
| <Y 12 and diploma/certificate | 125 | 1.35 (1.01, 1.81) | 1.40 (1.05, 1.87) | ||
| <Y 12 only | 21 | 0.99 (0.47, 2.10) | 1.20 (0.63, 2.30) | ||
| Parental occupation | 1283 | 0.60 | 0.02 | ||
| Managers/professionals | 867 | Ref | Ref | ||
| Trade/services | 247 | 1.00 (0.77, 1.29) | 1.14 (0.89, 1.46) | ||
| Clerical/sales | 133 | 0.95 (0.68, 1.32) | 1.52 (1.16, 1.98) | ||
| Laborer/driver/operators | 36 | 0.82 (0.41, 1.66) | 1.01 (0.55, 1.87) | ||
| Equivalized household income | 1242 | 0.75 | 0.05 | ||
| Highest quartile | 316 | Ref | Ref | ||
| Second quartile | 312 | 1.08 (0.81, 1.43) | 1.39 (1.03, 1.87) | ||
| Third quartile | 312 | 1.13 (0.85, 1.49) | 1.40 (1.04, 1.89) | ||
| Lowest quartile | 302 | 1.03 (0.77, 1.38) | 1.37 (1.01, 1.85) | ||
Values calculated using modified Poisson regression models adjusted for age, sex, body mass index z score, mean arterial blood pressure, pubertal category, minimal intima‐intima lumen diameter and exposure to passive smoking. Equivalized household income calculated as household (parental and other) weekly income divided by the square root of the number of family members, and split into quartiles. P values for trend across categories treat the exposure categories as a quasicontinuous variable.
Sensitivity Analyses
Analyses using imputed data in linear regression models gave results similar to those presented. Analyses using imputed data in modified Poisson regression models provided estimates with wider confidence intervals, which attenuated previously significant associations; however, the magnitude and direction of the point estimates remained similar.
In further separate analyses, we recategorized parental education into 3 groups and rescored parental occupation according to an alternative occupational ranking scale (AUSEI06). In a final sensitivity analysis, we included birth weight z scores to account for some potential postnatal factors. The results from these sensitivity analyses were similar to those presented.
Discussion
We provide the first evidence that the association between lower family SEP and subclinical atherosclerosis is already present in mid‐childhood. These findings are in keeping with the social gradients of traditional risk factors causally implicated in CVD pathogenesis, such as hypertension30 and obesity.31 The association between family SEP and carotid IMT was only mildly attenuated when accounting for neighborhood SEP and possible confounders and mediators, such as adiposity, blood pressure, and passive smoking exposure. In addition, longitudinal analyses suggest that SEP measured as early as ages 2 to 3 years is associated with subsequent subclinical atherosclerosis.
To our knowledge, this study is the first to examine the association between early life SEP and a widely used marker of subclinical atherosclerosis in mid‐childhood. Previous studies have reported that carotid IMT differs according to SEP in adults10, 11, 12, 13 and adolescents.14, 32 We provide evidence that lower SEP in very early childhood, and repeated measures of SEP throughout childhood, are associated with increased IMT at midchildhood. The absolute magnitude of the association is small—an effect size of 0.23 and 0.12 SD between highest and lowest family and neighborhood SEP quartiles, respectively, in cross‐sectional analyses—however, the association is consistent across different measures and components of SEP. Children in the lowest family SEP quartile have a 46% greater relative risk of having a maximum carotid IMT in the higher risk category, corresponding to an estimated vascular age at least 8 years older than their chronological age, according to published data on adolescent reference values.24 Such a trajectory persisting unchecked into adulthood may have clinical implications. Adults with carotid IMT in the top quartile (or evidence of carotid plaque) would have approximately twice the hazard for CVD, myocardial infarction, or stroke compared with counterparts with “normal” carotid IMT.33
Our study indicates that in children, family SEP and neighborhood SEP were associated with maximum carotid IMT but not with mean IMT. Maximum IMT has been suggested to be more relevant to cardiovascular risk7 and describes the nature of focal thickening of the intima–media early in the life course better than mean measures.34, 35 Prior studies among children have suggested using maximum carotid IMT measurements,34, 36 despite mean and maximum carotid IMT correlating strongly (r=0.76 in our cohort). A plausible explanation for different findings between mean and maximum carotid IMT is that the superior reproducibility of maximum IMT allowed an association to be detected, whereas noise in mean IMT measurement attenuated a true signal. Regardless, the mean carotid IMT regression estimates, although not statistically significant (P≤0.05), are consistent with the maximum carotid IMT estimates in direction and magnitude (Table 4). This adds weight to our overall conclusion that evidence shows a social gradient in carotid IMT measurement at 11 to 12 years of age.
Differences in associations with maximum carotid IMT between neighborhood and family SEP warrant further investigation. In our cohort, both measures were associated with IMT when analyzed separately, whereas family SEP seemed to have stronger explanatory power when analyzed simultaneously with neighborhood SEP. We suggest that family factors, for example, shared genetics and household environment, may be more important than neighborhood factors in the development of subclinical atherosclerosis. This is supported by recent evidence suggesting the causal pathways between neighborhood SEP and CVD involve SEP trends within individuals rather than the availability of resources.37 Further breakdown of the family SEP variable did not reveal clear differences in associations between carotid IMT and highest parental education, occupation, or household income (Table 5).
Further work is required to examine detailed mechanisms for the observed associations between SEP and carotid IMT. An association measurable by at least ages 2 to 3 years implies that early life heritable and/or environmental factors are likely to contribute. Their relative contribution and pathogenic mechanisms cannot be determined in the present study. CheckPoint did not have IMT measures from birth, but future studies could address whether associations between SEP and aortic IMT exist from birth. It is conceivable that differences may already exist in utero. Indeed, increased fatty streak formation has been observed in pregnancies of mothers with hypercholesterolemia.38 It is generally accepted that fetal environment partially determines cardiovascular risk,39 and this could partly represent intergenerational cellular memory mediated via epigenetic phenomena40 such as global hypermethylation of DNA secondary to chronic inflammation.41
Differential postnatal exposure to modifiable risk factors such as obesity, elevated blood pressure, and smoking over the life course are likely mechanisms by which SEP worsens atherosclerosis and increases risk for CVD.42, 43, 44, 45 Many of these exposures are initiated in childhood and adolescence, setting individuals on worse health trajectories later in life. It is plausible that early socioeconomic differences influence these risk factors, culminating in detectable changes in carotid IMT. Our sample demonstrated clear socioeconomic gradients in body mass index, blood pressure, and passive smoking exposure (Table 3); however, the association between SEP and IMT was independent of these factors. Other risk factors such as lipid profile, physical activity, and diet were not examined in this study, but it is unlikely that the present findings would be entirely attenuated by adjustment for these factors.46 In a comparative analysis of adult data, health behaviors accounted for socioeconomic differences in mortality only when the behaviors themselves were similarly patterned,45 suggesting that diet and physical activity are only a subset of potential mechanisms through which SEP affects health. Novel, nontraditional risk factors, such as increased childhood inflammatory burden, may contribute to the association between low SEP and atherosclerosis.47 Further detailed analyses of the intermediate pathways by which SEP influences early carotid IMT are warranted.
A number of limitations of our study warrant further consideration. The clinical consequences of increased carotid IMT in mid‐childhood are unknown. Associations between exposures such as obesity and cardiovascular risk may be modified in adolescence and early adulthood. Obese children who become thin adults, for example, have cardiovascular risk similar to those who were never obese.48 Data from ongoing studies measuring carotid IMT since young adulthood, such as those from the Cardiovascular Risk in Young Finns Study, may provide informative answers in the next decade,49 but withholding recommendations until longitudinal follow‐up of disease seems untenable in the context of the childhood obesity epidemic. Further studies are required to understand the relationship between childhood and adult IMT and the risk of CVD. Investigation of other markers of subclinical atherosclerosis, such as arterial distensibility and elasticity, or markers of microcirculation arteriosclerosis may be informative.
The CheckPoint sample is derived from an Australian population, limiting the generalizability of our conclusions to other ethnicities or cultural contexts. Sample characteristics such as Aboriginal or Torres Strait Islander status or speaking a language other than English at home did not substantially predict IMT, but the low proportion of these families in the sample meant that this study was not adequately powered to observe differences. Ethnic differences in carotid IMT50 may explain family SEP gradients in some contexts51; however, no evidence in the CheckPoint sample showed that ethnicity is a driving factor. Future studies comparing ethnically diverse groups including the Aboriginal population may provide further insight into this relationship. Moreover, the moderate response rate itself reduced the generalizability of our findings and introduced potential for selection bias. In particular, excluded participants had lower SEP than participants of the CheckPoint study overall; CheckPoint participants were relatively advantaged. The national mean for neighborhood SEP, for example, is set at 1000 (SD 100), and the lowest quartile of our sample had a mean of 1004 (SD 68). Across the wider national cohort, the carotid IMT differences are likely to be considerably greater; the undersampling of lower SEP families indicates the reported regression coefficients likely underestimate an association. The corollary is that even in this selective sample of advantaged families, evidence shows a family SEP gradient in carotid IMT.
Given the lack of clinical recommendations in children, we used published adult consensus statements to choose the 75th percentile cutoff point; this is used to indicate higher risk carotid IMT in adults22 and has been endorsed by the Association for European Pediatric Cardiology as a cutoff for abnormal IMT at younger ages.52 Nevertheless, this sample‐defined dichotomization should be interpreted with caution because it may not reflect the underlying population at the 75th percentile. If our sample were healthier than the general population, the 75th percentile cutoff would be at a lower level that inaccurately reflects underlying pathology. Longitudinal follow‐up is necessary to demonstrate the clinical relevance of this cutoff point.
We observed that SEP evaluated throughout childhood was associated with maximum carotid IMT at ages 11 to 12 years. Because SEP levels in early childhood are highly correlated with midchildhood SEP, it is difficult to know whether early childhood SEP per se is associated with IMT later in childhood or whether the finding is due to strong tracking of SEP.
Finally, our analyses are limited to the right carotid artery. A number of studies report an average of both arteries, whereas some studies report results from only the right artery.53 Some evidence suggests that left and right arteries have different IMTs; atherosclerosis tends to be more advanced in the left carotid artery compared with the right,54 possibly because of differences in left and right cerebral circulations. Even if the left carotid artery were thicker than the right by midchildhood, by measuring only 1 side, we were unlikely to introduce systematic biases.
In conclusion, we present evidence that family SEP and neighborhood SEP are associated with a marker of subclinical atherosclerosis by early childhood, independent of age, sex, puberty, passive smoking exposure, blood pressure, body size, and neighborhood SEP. Consistent evidence showed an association between SEP from early life and midchildhood carotid IMT. Future investigation should focus on how household factors influence early life health behaviors as potential mechanisms by which SEP alters early life trajectories of cardiovascular risk.
Appendix
The Child Health CheckPoint Investigator Group includes Tim Olds PhD, Louise Baur PhD, Lisa Gold PhD, Kate Lycett PhD, Jessica A. Kerr PhD, and Sarah Davies MPH.
Sources of Funding
This work has been supported to date by the National Health and Medical Research Council of Australia (1041352, 1109355), the Royal Children's Hospital Foundation (2014‐241), Murdoch Childrens Research Institute, the University of Melbourne, National Heart Foundation of Australia (100660), Financial Markets Foundation for Children (2014‐055) and Victorian Deaf Education Institute. The following authors were supported by the National Health and Medical Research Council of Australia: Clinical Postgraduate Research Scholarship (1114567) to Liu, Senior Research Fellowships (1046518) to Wake and (1064629) Burgner, Early Career Fellowship (1037449) and Career Development Fellowship (1111160) to Mensah. Liu is supported by an Australian Government Research Training Program Scholarship. Magnussen is supported by the National Heart Foundation of Australia Future Leader Fellowship (100849). Juonala is supported by the Federal Research Grant of Finland to Turku University Hospital, Finnish Cardiovascular Foundation, Juho Vainio Foundation, Sigrid Juselius Foundation, Maud Kuistila Foundation, the Paulo Foundation, and the Murdoch Childrens Research Institute (Dame Elizabeth Murdoch Fellowship). Wake is supported by Cure Kids, New Zealand. Research at the Murdoch Childrens Research Institute is supported by the Victorian Government's Operational Infrastructure Program. The funding bodies did not play any role in the study. This article uses unit record data from the Longitudinal Study of Australian Children. The study is conducted in partnership between the Department of Social Services (DSS), the Australian Institute of Family Studies (AIFS) and the Australian Bureau of Statistics (ABS). The findings and views reported in this article are those of the author and should not be attributed to DSS, AIFS or the ABS.
Disclosures
None.
Acknowledgments
We would like to acknowledge Denise Becker for her contribution to the carotid IMT reliability modelling and statistics, Greta Goldsmith for her significant contribution to data analysis and processing, and Josh Muller for his contribution to data collation and cleaning. Some study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools. REDCap is a secure, Web‐based application designed to support data capture for research studies.
(J Am Heart Assoc. 2017;6:e005925 DOI: 10.1161/JAHA.117.005925.)28862928
References
- 1. Mackenbach JP, Kulhanova I, Artnik B, Bopp M, Borrell C, Clemens T, Costa G, Dibben C, Kalediene R, Lundberg O, Martikainen P, Menvielle G, Ostergren O, Prochorskas R, Rodriguez‐Sanz M, Strand BH, Looman CW, de Gelder R. Changes in mortality inequalities over two decades: register based study of European countries. BMJ. 2016;353:i1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooper RS. Social inequality, ethnicity and cardiovascular disease. Int J Epidemiol. 2001;30(suppl 1):S48–S52. [DOI] [PubMed] [Google Scholar]
- 3. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37:3232–3245. [DOI] [PubMed] [Google Scholar]
- 4. Waters E, de Silva‐Sanigorski A, Burford Belinda J, Brown T, Campbell Karen J, Gao Y, Armstrong R, Prosser L, Summerbell Carolyn D. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2011;12:CD001871. [DOI] [PubMed] [Google Scholar]
- 5. Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, Jacobson M, Mahoney L, Mietus‐Snyder M, Rocchini A, Steinberger J, McCrindle B; American Heart Association Atherosclerosis Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young . Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. [DOI] [PubMed] [Google Scholar]
- 6. Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O'Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML. Common carotid intima‐media thickness measurements in cardiovascular risk prediction: a meta‐analysis. JAMA. 2012;308:796–803. [DOI] [PubMed] [Google Scholar]
- 7. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation. 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 8. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. [DOI] [PubMed] [Google Scholar]
- 9. Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, Chen W, Srinivasan SR, Daniels SR, Kahonen M, Laitinen T, Taittonen L, Berenson GS, Viikari JS, Raitakari OT. Influence of age on associations between childhood risk factors and carotid intima‐media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122:2514–2520. [DOI] [PubMed] [Google Scholar]
- 10. Lynch J, Kaplan GA, Salonen R, Cohen RD, Salonen JT. Socioeconomic status and carotid atherosclerosis. Circulation. 1995;92:1786–1792. [DOI] [PubMed] [Google Scholar]
- 11. Petersen KL, Bleil ME, McCaffery J, Mackey RH, Sutton‐Tyrrell K, Muldoon MF, Manuck SB. Community socioeconomic status is associated with carotid artery atherosclerosis in untreated, hypertensive men. Am J Hypertens. 2006;19:560–566. [DOI] [PubMed] [Google Scholar]
- 12. Carson AP, Rose KM, Catellier DJ, Kaufman JS, Wyatt SB, Diez‐Roux AV, Heiss G. Cumulative socioeconomic status across the life course and subclinical atherosclerosis. Ann Epidemiol. 2007;17:296–303. [DOI] [PubMed] [Google Scholar]
- 13. Nash SD, Cruickshanks KJ, Klein R, Klein BE, Nieto FJ, Ryff CD, Krantz EM, Shubert CR, Nondahl DM, Acher CW. Socioeconomic status and subclinical atherosclerosis in older adults. Prev Med. 2011;52:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lamotte C, Iliescu C, Beghin L, Salleron J, Gonzalez‐Gross M, Marcos A, De Henauw S, Moreno LA, Libersa C, Gottrand F. Association of socioeconomic status, truncal fat and sICAM‐1 with carotid intima‐media thickness in adolescents: the HELENA study. Atherosclerosis. 2013;228:460–465. [DOI] [PubMed] [Google Scholar]
- 15. Winkleby MA, Cubbin C. Influence of individual and neighbourhood socioeconomic status on mortality among black, Mexican‐American, and white women and men in the United States. J Epidemiol Community Health. 2003;57:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Australian Bureau of Statistics; Census of population and housing: socio‐economic indexes for areas (SEIFA). 2011. Cat. No. 2033.0.55.001. 2011. [Google Scholar]
- 17. Blakemore T, Strazdins L, Gibbings J. Measuring family socioeconomic position. Aust Soc Policy. 2009;8:121–168. [Google Scholar]
- 18. Berthelsen D, Walker S. Parents’ involvement in their children's education. Fam Matters. 2008;79:34–41. [Google Scholar]
- 19. Trewin D, Trewin DJ, Pink BN. ANZSCO: Australian and New Zealand standard classification of occupations. Australian Bureau of Statistics/Statistics New Zealand; 2006.
- 20. McMillan J, Beavis A, Jones FL. The AUSEI06 a new socioeconomic index for Australia. J Sociol. 2009;45:123–149. [Google Scholar]
- 21. Mullan K, Daragavona G, Baker K. Imputing income in the Longitudinal Study of Australian Children, LSAC (technical paper no. 12). Melbourne: Australian Institute of Family Studies; 2015. [Google Scholar]
- 22. Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS; American Society of Echocardiography Carotid Intima‐Media Thickness Task Force . Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima‐Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111; quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 23. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima‐media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th Watching the Risk Symposia, at the 13th, 15th and 20th European Stroke conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doyon A, Kracht D, Bayazit AK, Deveci M, Duzova A, Krmar RT, Litwin M, Niemirska A, Oguz B, Schmidt BM, Sozeri B, Querfeld U, Melk A, Schaefer F, Wuhl E; Consortium CS . Carotid artery intima‐media thickness and distensibility in children and adolescents: reference values and role of body dimensions. Hypertension. 2013;62:550–556. [DOI] [PubMed] [Google Scholar]
- 25. Bond L, Clements J, Bertalli N, Evans‐Whipp T, McMorris BJ, Patton GC, Toumbourou JW, Catalano RF. A comparison of self‐reported puberty using the pubertal development scale and the sexual maturation scale in a school‐based epidemiologic survey. J Adolesc. 2006;29:709–720. [DOI] [PubMed] [Google Scholar]
- 26. Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 28. van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. [DOI] [PubMed] [Google Scholar]
- 29. Dobbins TA, Sullivan EA, Roberts CL, Simpson JM. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med J Aust. 2012;197:291–294. [DOI] [PubMed] [Google Scholar]
- 30. Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal‐medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics. 2003;111:61–66. [DOI] [PubMed] [Google Scholar]
- 31. Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, Romano ML, Panico S, Rubba P, Trevisan M. Increased carotid intima‐media thickness and stiffness in obese children. Diabetes Care. 2004;27:2506–2508. [DOI] [PubMed] [Google Scholar]
- 32. Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med. 2009;68:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima‐media thickness for cardiovascular disease prediction in the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skilton MR, Nakhla S, Ayer JG, Harmer JA, Toelle BG, Leeder SR, Jones G, Marks GB, Celermajer DS; Childhood Asthma Prevention Study group . Telomere length in early childhood: early life risk factors and association with carotid intima‐media thickness in later childhood. Eur J Prev Cardiol. 2016;23:1086–1092. [DOI] [PubMed] [Google Scholar]
- 35. Napoli C, Glass CK, Witztum JL, Deutsch R, D'Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354:1234–1241. [DOI] [PubMed] [Google Scholar]
- 36. Skilton MR, Evans N, Griffiths KA, Harmer JA, Celermajer DS. Aortic wall thickness in newborns with intrauterine growth restriction. Lancet. 2005;365:1484–1486. [DOI] [PubMed] [Google Scholar]
- 37. Calling S, Li X, Kawakami N, Hamano T, Sundquist K. Impact of neighborhood resources on cardiovascular disease: a nationwide six‐year follow‐up. BMC Public Health. 2016;16:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Napoli C, D'Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. [DOI] [PubMed] [Google Scholar]
- 40. Wierda RJ, Geutskens SB, Jukema JW, Quax PH, van den Elsen PJ. Epigenetics in atherosclerosis and inflammation. J Cell Mol Med. 2010;14:1225–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stenvinkel P, Karimi M, Johansson S, Axelsson J, Suliman M, Lindholm B, Heimburger O, Barany P, Alvestrand A, Nordfors L, Qureshi AR, Ekstrom TJ, Schalling M. Impact of inflammation on epigenetic DNA methylation—a novel risk factor for cardiovascular disease? J Intern Med. 2007;261:488–499. [DOI] [PubMed] [Google Scholar]
- 42. Marmot MG, Shipley MJ, Hemingway H, Head J, Brunner EJ. Biological and behavioural explanations of social inequalities in coronary heart disease: the Whitehall II study. Diabetologia. 2008;51:1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blane D, Hart CL, Smith GD, Gillis CR, Hole DJ, Hawthorne VM. Association of cardiovascular disease risk factors with socioeconomic position during childhood and during adulthood. BMJ. 1996;313:1434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Albert MA, Glynn RJ, Buring J, Ridker PM. Impact of traditional and novel risk factors on the relationship between socioeconomic status and incident cardiovascular events. Circulation. 2006;114:2619–2626. [DOI] [PubMed] [Google Scholar]
- 45. Stringhini S, Dugravot A, Shipley M, Goldberg M, Zins M, Kivimaki M, Marmot M, Sabia S, Singh‐Manoux A. Health behaviours, socioeconomic status, and mortality: further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Med. 2011;8:e1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kestila P, Magnussen CG, Viikari JS, Kahonen M, Hutri‐Kahonen N, Taittonen L, Jula A, Loo BM, Pietikainen M, Jokinen E, Lehtimaki T, Kivimaki M, Juonala M, Raitakari OT. Socioeconomic status, cardiovascular risk factors, and subclinical atherosclerosis in young adults: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2012;32:815–821. [DOI] [PubMed] [Google Scholar]
- 47. Lawson JS. Multiple infectious agents and the origins of atherosclerotic coronary artery disease. Front Cardiovasc Med. 2016;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. [DOI] [PubMed] [Google Scholar]
- 49. Juhola J, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Kahonen M, Taittonen L, Urbina E, Viikari JS, Dwyer T, Raitakari OT, Juonala M. Combined effects of child and adult elevated blood pressure on subclinical atherosclerosis: the International Childhood Cardiovascular Cohort Consortium. Circulation. 2013;128:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. D'Agostino RB Jr, Burke G, O'Leary D, Rewers M, Selby J, Savage PJ, Saad MF, Bergman RN, Howard G, Wagenknecht L, Haffner SM. Ethnic differences in carotid wall thickness. The Insulin Resistance Atherosclerosis Study. Stroke. 1996;27:1744–1749. [DOI] [PubMed] [Google Scholar]
- 51. Ranjit N, Diez‐Roux AV, Chambless L, Jacobs DR Jr, Nieto FJ, Szklo M. Socioeconomic differences in progression of carotid intima‐media thickness in the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol. 2006;26:411–416. [DOI] [PubMed] [Google Scholar]
- 52. Dalla Pozza R, Ehringer‐Schetitska D, Fritsch P, Jokinen E, Petropoulos A, Oberhoffer R; Association for European Paediatric Cardiology Working Group Cardiovascular Prevention . Intima media thickness measurement in children: a statement from the Association for European Paediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis. 2015;238:380–387. [DOI] [PubMed] [Google Scholar]
- 53. Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. [DOI] [PubMed] [Google Scholar]
- 54. Luo X, Yang Y, Cao T, Li Z. Differences in left and right carotid intima‐media thickness and the associated risk factors. Clin Radiol. 2011;66:393–398. [DOI] [PubMed] [Google Scholar]


