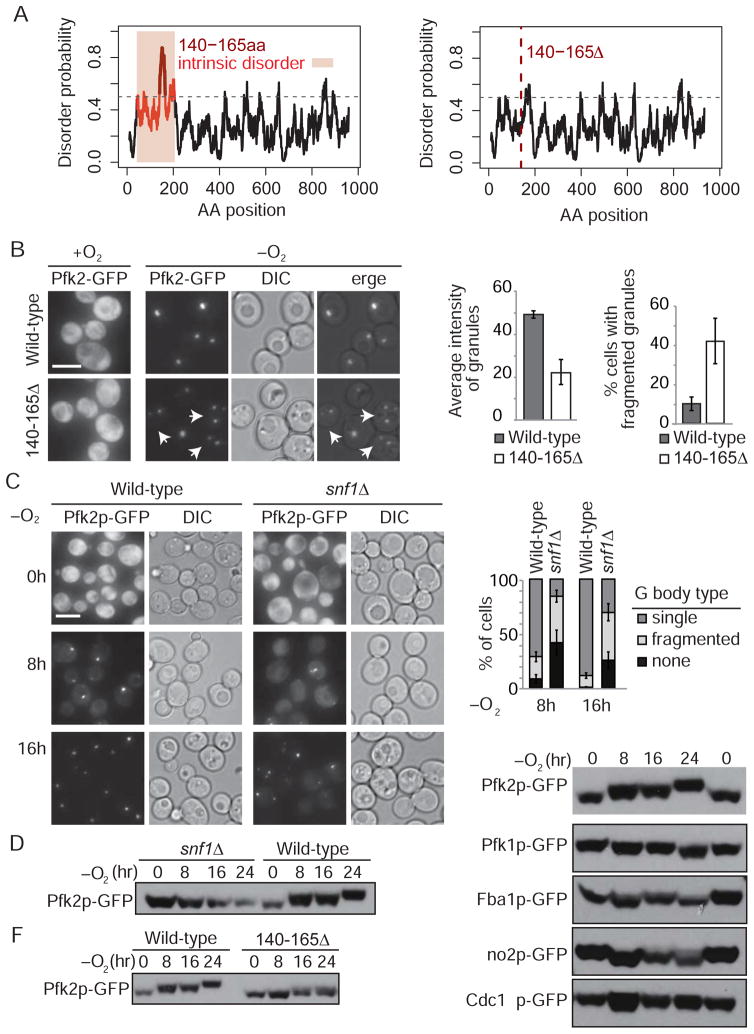
Figure 3. G Body formation is Snf1-dependent.
(A) Pfk2p intrinsically disordered domain (IDR) as predicted by IUPred in wild-type and 140–165Δ Pfk2p. (B) Cells expressing GFP-tagged 140–165Δ Pfk2p and subjected to 16 h hypoxic treatment contain multiple fragmented granules (indicated by arrows) (p<0.05 versus wild-type by student’s t-test). Average intensity of granules measured by ImageJ (p<0.01 versus wild-type). Error bars represent SEM for at least three independent experiments. (C) A majority of snf1Δ cells display no or fragmented Pfk2p-GFP granules after 16 h hypoxia. Error bars represent SEM for more than three independent experiments. At 8 h, t-test comparing snf1Δ to wild-type, p<0.05 for cells without granules and cells with more than one granule and p<10−4 for cells with one granule. At 16 h, p<0.05 for cells without granules, p<0.01 for cells with more than one granule and cells with one granule. (D–F) Phosphorylation of Pfk2p-GFP is compromised in snf1Δ cells and Pfk2p is phosphorylated at its IDR. Cells expressing GFP-tagged glycolytic enzymes or Pfk2pΔ140–165 were collected after specified durations of anaerobic treatment. Protein extracts were separated by Mn2+-Phos-tag SDS-PAGE and subjected to immunoblotting using a GFP antibody. The images in D and F are from the same blot with the same wild-type control. All scale bars 5 μm. [See also figure S6]