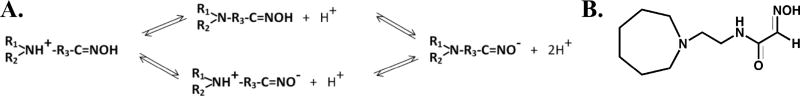
Scheme 1.
(A) Ionization equilibria of the hydroxyiminoacetamidoalkyl cyclic amines showing the transition from cations to anions to proceed through zwitterionic and neutral species. The oximate-oxime and amine protonation in the azepine ring have nearly identical pKa’s ~8.8. The neutral species facilitates crossing the blood-brain barrier and oral bioavailability, while the cation will be attracted to the active center gorge and in solution the anion is the nucleophilic, general base catalyst. (B) The lead structure, RS 194B of over 100 congeners synthesized, is shown on the right.