Abstract
Context:
Obstructive sleep apnea (OSA) is associated with diabetes and cardiovascular disease. This association may be related to metabolic changes that transpire during sleep in OSA.
Objective:
To examine the impact of OSA, elicited by cessation of continuous positive airway pressure (CPAP), on frequently sampled nocturnal metabolic markers including plasma free fatty acids (FFAs), glucose, insulin, triglycerides (TGs), cortisol, and lactate, as well as glucose production, oral glucose tolerance, blood pressure (BP), endothelial function, cholesterol, and high-sensitivity C-reactive protein (hsCRP).
Design and Setting:
Randomized crossover trial of CPAP vs CPAP withdrawal.
Patients:
Thirty-one patients with moderate to severe OSA acclimated to CPAP.
Intervention:
Patients underwent attended polysomnography while sleeping with therapeutic CPAP, or after CPAP withdrawal, in random order. Venous blood was sampled at ∼20-minute intervals on both nights. In 11 patients, we assessed glucose kinetics with an infusion of 6,6-[2H2]glucose.
Results:
CPAP withdrawal caused recurrence of OSA associated with hypoxemia, sleep disruption, and heart rate (HR) elevation. CPAP withdrawal dynamically increased nocturnal FFA (P = 0.007), glucose (P = 0.028), and cortisol (P = 0.037), in proportion to respiratory event frequency, HR elevation, or sleep fragmentation. Diabetes predisposed to glucose elevation. CPAP withdrawal also increased systolic BP (P = 0.017) and augmentation index (P = 0.008), but did not affect insulin, TGs, glucose production, oral glucose tolerance, cholesterol, or hsCRP.
Conclusion:
OSA recurrence during CPAP withdrawal increases FFA and glucose during sleep, associated with sympathetic and adrenocortical activation. Recurring exposure to these metabolic changes may foster diabetes and cardiovascular disease.
We studied the overnight metabolic profile of patients during sleep, in the presence or absence of sleep apnea. Sleep apnea caused dynamic elevations of plasma FFA and glucose.
Obstructive sleep apnea (OSA) is a prevalent sleep-induced breathing disorder associated with diabetes (1) and cardiovascular disease (CVD) (2). It is unclear whether OSA is a cause, consequence, or marker of cardiometabolic dysfunction. Most metabolic studies in OSA patients have collected data during wakefulness, and report inconclusive effects of OSA or its treatment with continuous positive airway pressure (CPAP). However, this approach can only assess the “aftermath” of OSA, not the sleep period when OSA is occurring. Because sleep comprises one third of the human lifespan, altered metabolism during this period may have substantial health implications.
Plasma free fatty acids (FFAs), glucose, insulin, triglycerides (TGs), and cortisol have established roles in diabetes and CVD. These substances also have the potential to fluctuate during sleep and normalize after awakening. For example, we previously showed that OSA increases plasma FFA in heart failure patients shortly after sleep onset (3). Similarly, exposure of mice to intermittent hypoxia simulating OSA caused lipolysis and hyperglycemia within minutes, which normalized during recovery (4–6). Acute hypoxia in humans can increase plasma glucose (7) and FFA (8). These findings indicate that OSA is an episodic stressor, best studied with a frequent blood-sampling approach during sleep.
In this study, we compared treated vs untreated OSA in the same patients using CPAP withdrawal (9). This approach confers two major advantages over starting CPAP in treatment-naïve patients. First, this avoids enrolling patients who will not ultimately use CPAP, a common clinical problem (10). Second, as CPAP is discontinued for only a few days, we can include patients with severe OSA, who would be excluded from randomized trials. Theoretically, these represent the very patients who are most vulnerable to consequences of OSA, and the most mechanistically informative. We admitted CPAP-acclimatized OSA patients to the sleep laboratory for 2 nights of polysomnography (PSG) with simultaneous detailed serial metabolic assessment. One night, patients slept with CPAP, while on the other night, they slept without CPAP, after stopping CPAP for 2 nights. During sleep, we sampled blood at 20-minute intervals to measure FFA, glucose, insulin, TG, cortisol, and lactate. We hypothesized that OSA dynamically increases FFA and glucose and that OSA increases endogenous glucose output, as determined by the rate of glucose appearance (Ra glucose) using a primed continuous infusion of 6,6-[2H2] glucose. Finally, we assessed blood pressure (BP), endothelial function via EndoPAT (Intamar Medical, Caesarea, Israel), cholesterol, and high-sensitivity C-reactive protein (hsCRP) because metabolic changes can also influence these parameters.
Materials and Methods
This study was approved by the Johns Hopkins Institutional Review Board. Patients with OSA were recruited from our Sleep Disorders Center. Inclusion criteria included age ≥20 and ≤75 years old, history of OSA with apnea-hypopnea index (AHI) ≥20), and accustomed to CPAP use. Exclusion criteria included uncontrolled hypertension with systolic BP >170 mm Hg or diastolic BP >110 mm Hg, congestive heart failure, use of clonidine or nicotinic acid, diabetes requiring use of insulin, and pregnancy. Postenrollment, if patients slept poorly in the laboratory (sleep efficiency <50%), their data were excluded from analysis.
Study design
This was a single-center, randomized crossover trial of OSA (acute CPAP withdrawal) vs CPAP. Patients underwent PSG with nocturnal venous blood sampling while treated with CPAP, or without CPAP, separated by a 1- to 4-week washout. Because OSA may not recur immediately during CPAP withdrawal (11), patients discontinued CPAP for 2 nights preceding their OSA visit. We asked them to continue their usual activities, diet, and medication for both visits.
PSG
On each evening, from 5:30 to 6:30 pm, patients ate a research dinner comprised of 30% fat, 50% carbohydrate, and 20% protein with calories based on the Mifflin–St. Jeor formula. Two peripheral IVs were placed, one for infusion of tracer, and the other for sampling. IV tubing was extended to an adjacent room through a VAMP Plus® (Edwards Lifesciences, Irvine, CA) system. Attended PSG was performed from 10:40 pm until 6:40 am and monitored electroencephalography, electrooculography, oximetry, respiratory effort, and transcutaneous CO2 (tcCO2; Radiometer TCM-4). We staged sleep and scored OSA events by American Academy of Sleep Medicine guidelines as previously published (11).
Blood sampling, tracer infusion, and metabolic assays
Venous samples were obtained at 9:00 pm, 9:40 pm, 10:00 pm, 10:40 pm (lights turned out), 11:20 pm, and every 20 minutes thereafter until 6:40 am when patients were awakened (Supplemental Fig. 1 (212KB, docx) ). In eleven nondiabetics, we administered a primed (30 µmol/kg, at 9:05 pm) continuous (20 µmol/kg/h) infusion of 6,6-[2H2] glucose (Cambridge Isotopes Laboratory, Andover, MA) throughout sleep. This tracer was selected as it does not recycle into plasma after gluconeogenesis, which can underestimate glucose production (12). Tracer was solubilized to 3 M, in 0.9% NaCl, filtered through a 0.22-μm filter, and tested for sterility. Tracer was frozen, then thawed and diluted to 0.3 M on the evening of infusion. Infusate was enriched at 98.6 mol % excess. Determination of isotopic enrichment was performed by gas chromatography/mass spectroscopy (Metabolic Solutions, Nashua, NH). Enrichment of glucose (E) was expressed as mol % excess = TTR/(1 + TTR), where TTR is ratio of tracer to tracee. Rates of glucose appearance (Ra), disappearance (Rd), and metabolic clearance rate (MCR) were calculated using Steele’s equations (13):
where F is the infusion rate of 6,6-[2H2] glucose; pV is the effective volume of distribution for glucose (40 mg/kg); C1 and C2 are plasma glucose concentrations at times t1 and t2, respectively; E1 and E2 are plasma enrichment of 6,6-[2H2] glucose at times t1 and t2, respectively. From plasma, we measured FFA and TGs (Wako, Richmond, VA), insulin (Linco, Saint Charles, MO), glucose and lactate (Eton Bioscience, San Diego, CA), cortisol (Alpco, Salem, NH), and lipid panel (Alere Cholestech LDX Analyzer, Providence, RI).
Morning procedures
EndoPAT (Itamar Medical), dual-energy X-ray absorptiometry scanning, and a 2-hour 75-g oral glucose tolerance test (OGTT) were performed. For EndoPAT, a BP cuff was placed on the nondominant arm while the contralateral arm was used as control. Finger-pulse wave amplitude was measured for 5 minutes, during 5-minute occlusion of the brachial artery by inflation of the cuff to 80 mm Hg above systolic BP, and for 5 minutes after cuff release. The reactive hyperemia index (RHI) and the augmentation index normalized to the heart rate (HR) at 75 beats/min were derived by EndoPAT-2000 software.
Statistical analysis
Our primary outcomes were real-time (nocturnal) and cumulative (morning) cardiometabolic disturbances caused by resumption of OSA. Because patients served as their own control, data were analyzed with mixed effects models with random intercepts to account for intersubject differences in OSA severity and metabolism (14). In the primary analysis, OSA exposure was the fixed factor. To investigate the relationship between sleep physiology and metabolic disturbances, a second analysis modeled metabolic outcomes as functions of physiologic parameters, summarized over the 20-minute window preceding the blood sample. We incorporated iterative time lags for some parameters to account for potential delayed effects. For single time-point outcomes, we used paired t tests for comparisons within patients, and unpaired t tests between patients. We also examined interrelated changes in metabolism and sleep between nights by Pearson correlation. Two-sided P values < 0.05 were considered statistically significant. All analyses were performed using STATA version 12.0.
Results
Baseline characteristics of patients
We enrolled 42 patients to the study. Data from 11 patients was excluded (n = 6, sleep efficiency <50%; n = 3, IV failure; two did not return after one visit). This left 31 patients for analysis. Table 1 shows the clinical characteristics of the patients. The average age was 50.8 years old and the average body mass index was 37.4 kg/m2. Approximately two-thirds of the group was male, one-fourth had a history of non-insulin-dependent diabetes, and more than half had a history of hypertension and hyperlipidemia. Some patients were taking statins (32.3%), metformin (12.9%), or beta-blockers (9.7%). The cohort was 22.6% African American, 9.7% Asian, 64.5% Caucasian, and 3.2% Hispanic.
Table 1.
Clinical Characteristics of Study Patients (n = 31)
| Variable | Mean ± Standard Error of the Mean (%) |
|---|---|
| Age, y | 50.8 ± 1.9 |
| Sex, n (%) | |
| Male | 21 (67.7) |
| Female | 10 (32.3) |
| Race | |
| African American, n (%) | 7 (22.6) |
| Asian, n (%) | 3 (9.7) |
| Caucasian, n (%) | 20 (64.5) |
| Hispanic, n (%) | 1 (3.2) |
| Body composition | |
| Body mass index, kg/m2 | 37.4 ± 1.3 |
| Waist: hip ratio | 0.98 ± 0.015 |
| Fat mass, % | 39.9 ± 2.9 |
| Lean mass, % | 56.9 ± 2.8 |
| Comorbidities and medications | |
| Diabetes, n (%) | 7 (22.5) |
| HbA1c, % | 6.37 ± 0.24 |
| Hypertension, n (%) | 17 (54.8) |
| Hyperlipidemia, n (%) | 18 (58.1) |
| Metformin, n (%) | 4 (12.9) |
| Statin, n (%) | 10 (32.3) |
| Beta-blocker, n (%) | 3 (9.7) |
| Smoker, n (%) | 2 (6.4) |
Abbreviation: HbA1c, hemoglobin A1c.
Effects of CPAP withdrawal on sleep and physiology
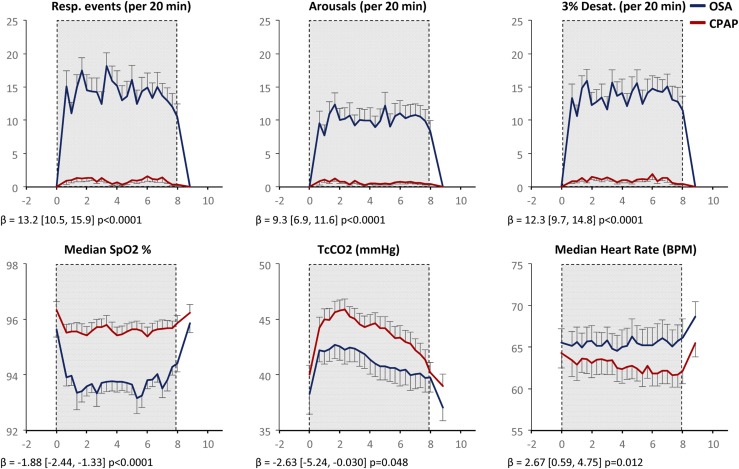
Supplemental Table 1 (212KB, docx) shows summarized OSA and sleep parameters on CPAP and OSA nights. CPAP suppressed OSA, whereas CPAP withdrawal caused recurrence of OSA (average AHI of 60.7). As expected, CPAP withdrawal increased the frequency of ≥3% oxygen desaturations (oxygen desaturation index), minutes spent with oxygen saturation (SpO2) <90% (T90%), and sleep arousals. CPAP withdrawal increased stage N1 and reduced stage N3 and REM sleep (Supplemental Table 1 (212KB, docx) , Supplemental Fig. 2 (212KB, docx) ). Figure 1 shows the time course of respiratory events (apneas + hypopneas), sleep arousals, ≥3% oxygen desaturations, and HR, as well as reduced SpO2 and tcCO2 during CPAP withdrawal. The unexpected reduction in tcCO2 may be due to vasoconstriction (15) or loss of REM sleep where CO2 levels are highest.
Figure 1.
Effect of CPAP withdrawal on sleep physiology. Data are plotted as mean ± standard error of the mean (n = 31) at 20-minute intervals. OSA data are shown in blue; CPAP data are shown in red. The shaded region, from 11 pm to 7 am, denotes the sleep/lights out period. Respiratory events = (apneas + hypopneas) in preceding 20 minutes. β coefficients and P values reflect differences between OSA and CPAP using mixed effects models. BPM, beats per minute.
Effects of CPAP withdrawal on metabolism
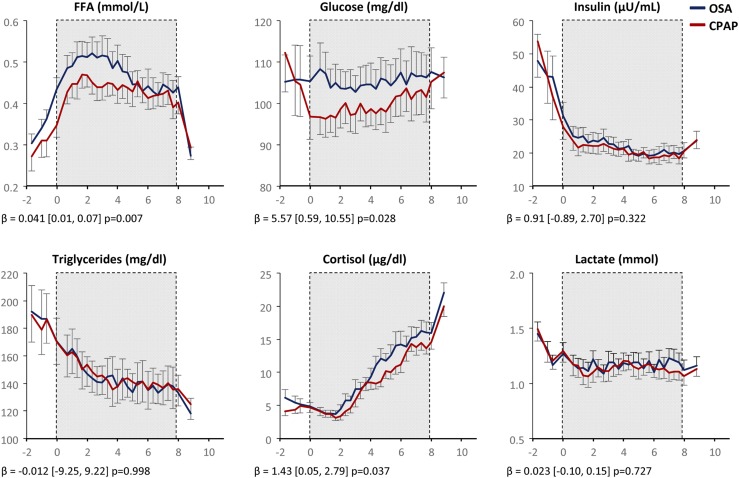
Preceding sleep, on both CPAP therapy and withdrawal nights, we observed a rise in FFA and a fall in glucose, insulin, and TG levels consistent with the late postprandial period (Fig. 2). As patients fell asleep and were exposed to OSA, metabolic profiles diverged. CPAP withdrawal increased nocturnal plasma FFA [β = 0.041 mmol/L, (95% CI, 0.01, 0.07), P = 0.007], glucose [β = 5.57 mg/dL, (95% CI, 0.59, 10.55), P = 0.028], and cortisol [β = 1.43 µg/dL, (95% CI, 0.05, 2.79), P = 0.037]. Insulin did not increase overall, but increased proportionately with glucose. CPAP withdrawal did not increase TG or lactate. Outcomes were not significantly altered by adjustments for visit order, body mass index, or fat mass (not shown). Metabolic responses to OSA were heterogeneous. In Supplemental Fig. 3 (212KB, docx) , we illustrate striking overnight metabolic changes dynamically occurring in a “responder” and minimal metabolic changes in a “nonresponder,” despite exposure to a similar severity of OSA.
Figure 2.
Effect of CPAP withdrawal on nocturnal metabolic profiles. OSA data are in blue; CPAP data are in red. Values are plotted as mean ± standard error of the mean (n = 31). The shaded region from 11 pm to 7 am denotes the sleep/lights out period. β coefficients and P values reflect differences between OSA and CPAP using mixed effects models, and do not include the OGTT period.
Predictors of metabolic responses to OSA
Next, we examined the dynamic impact of sleep physiology on metabolism, using 46 time points per patient (23 time points on the CPAP night and 23 time points on the OSA night). We hypothesized that metabolism at any given time point would be affected by the preceding frequency of respiratory events, degree of hypoxemia, sympathetic activity (HR), adrenocortical activity (cortisol), and sleep architecture. We tested this hypothesis with mixed-effects models where metabolic outcomes were regressed against preceding sleep physiology (Table 2, standardized beta coefficients in Supplemental Table 2 (212KB, docx) ). In fact, each respiratory event in the preceding 20 minutes was associated with an increase in FFA by 0.002 mmol/L (P = 0.004) and of glucose by 0.24 mg/dL (P = 0.018). As expected from collinearity of OSA severity variables, we obtained similar results using metrics of hypoxemia as predictors (median SpO2% or number of desaturations). The HR antecedent to blood draws was highly predictive of metabolic disturbances. A 1-beat/min increase of HR was associated with an increase in FFA of 0.003 mmol/L (P = 0.030), in glucose of 1.01 mg/dL (P = 0.008), in insulin of 0.22 µU/mL (P = 0.007), and in lactate of 0.016 mmol/L (P = 0.001). Sleep architecture affected cortisol and lactate levels: cortisol decreased with stage N2 or N3 sleep and increased as a function of stage N1 or wakefulness. Lactate decreased with stage N2 sleep.
Table 2.
Dynamic Predictors of Metabolism During Sleep
| Outcome | Respiratory Eventsa |
Median SpO2, % |
3% Desaturations |
Median HR, beats/min |
Cortisol |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | CI | β | CI | β | CI | β | CI | β | CI | |
| FFA, mmol/L | 0.002c | 0.001 to 0.003 | −0.010b | −0.02 to −0.00 | 0.002b | 0.001 to 0.003 | 0.003b | 0.00 to 0.01 | 0.00 | −0.00 to 0.00 |
| Glucose, mg/dL | 0.235b | 0.04 to 0.43 | −0.929c | −1.54 to −0.32 | 0.233b | 0.04 to 0.42 | 1.010c | 0.26 to 1.76 | 0.557d | 0.25 to 0.87 |
| Insulin, µU/mL | 0.003 | −0.05 to 0.06 | −0.034 | −0.35 to 0.28 | −0.011 | −0.08 to 0.06 | 0.224c | 0.06 to 0.39 | −0.195 | −0.40 to 0.01 |
| Lactate, mmol/L | 0.001 | −0.00 to 0.01 | −0.005 | −0.03 to 0.02 | 0.00 | −0.01 to 0.00 | 0.016d | 0.01 to 0.03 | 0.005 | −0.00 to 0.01 |
| Cortisol, µg/dL | 0.031 | −0.10 to 0.16 | −0.102 | −0.46 to 0.26 | 0.045 | −0.05 to 0.14 | 0.185 | −0.18 to 0.55 | ||
| N1,e % | N2, % | N3, % | REM, % | Wake, % | ||||||
| Outcome | β | CI | β | CI | β | CI | β | CI | β | CI |
| FFA, mmol/L | 0.00 | −0.00 to 0.00 | 0.00 | −0.00 to 0.00 | 0.00 | −0.00 to 0.00 | 0.00 | −0.00 to 0.00 | 0.00 | −0.00 to 0.00 |
| Glucose, mg/dL | 0.115 | −0.01 to 0.24 | −0.02 | −0.05 to 0.01 | −0.036 | −0.11 to 0.03 | −0.019 | −0.04 to 0.00 | 0.035 | −0.01 to 0.08 |
| Insulin, µU/mL | −0.003 | −0.04 to 0.03 | −0.005 | −0.01 to 0.00 | 0.033c | 0.01 to 0.06 | −0.007 | −0.02 to 0.01 | −0.001 | −0.02 to 0.01 |
| Lactate, mmol/L | 0.002 | −0.00 to 0.01 | −0.001d | −0.00 to −0.00 | 0.00 | −0.00 to 0.00 | 0.00 | −0.00 to 0.00 | 0.00 | −0.00 to 0.00 |
| Cortisol, µg/dL | 0.110b | 0.02 to 0.20 | −0.034c | −0.06 to −0.01 | −0.063d | −0.08 to −0.04 | 0.002 | −0.02 to 0.02 | 0.050d | 0.03 to 0.07 |
β coefficients are obtained from mixed-effects models, in which each metabolic outcome was regressed against respiratory events, hypoxia parameters, HR, cortisol, or sleep-stage composition in the 20 minutes immediately preceding the blood draw. Standardized β coefficients for these regressions appear in Supplemental Table 2.
Abbreviation: CI, confidence interval.
Respiratory events = number of (apneas + hypopneas).
P < 0.05.
P < 0.01.
P < 0.001.
N1, N2, and N3 refer to non-REM sleep stages 1, 2, and 3, respectively.
To assess more stringently within-night metabolic responses to OSA, we also examined the preceding relationships using data from only the OSA night (Supplemental Table 3 (212KB, docx) ). This analysis confirms that FFA, glucose, and cortisol levels fluctuate in real-time following changes in sleep physiology. In addition, metabolic changes were associated with respiratory events or HR changes up to 80 minutes prior, in a decaying fashion (Supplemental Table 4 (212KB, docx) ). We also explored patient characteristics associated with metabolic responses to CPAP withdrawal. First, we separately examined patients with diabetes and nondiabetics (Supplemental Figs. 4 and 5 (212KB, docx) ). CPAP withdrawal led to a similar AHI in patients with diabetes and nondiabetics (AHI off CPAP = 64.4 ± 12.6, 59.8 ± 6.4, respectively). The glucose profile was markedly increased by OSA in patients with diabetes during sleep (β = 17.2 mg/dL [95% CI, 0.13, 34.6], P = 0.042), whereas OSA did not increase glucose in nondiabetics (P = 0.139). Second, we compared metabolic “responders” to “nonresponders” based on a ≥10% increase in FFA or glucose during CPAP withdrawal (Supplemental Table 5 (212KB, docx) ). Based on these thresholds, we identified 14 of 31 (45.2%) FFA responders and 11 of 31 (35.5%) glucose responders. We did not observe distinctive clinical features of FFA responders, other than a trend toward TG and cholesterol elevation. Glucose responders tended to be patients with diabetes (P = 0.092) who exhibited concurrent elevation of insulin (P = 0.007), cortisol (P = 0.092), and hsCRP (P = 0.085). Responders did not differ with respect to changes in morning OGTT, vascular outcomes, or BP (not shown). Interestingly, “summary” metrics of sleep apnea severity (e.g., AHI) were not significantly different between responders and nonresponders. This reflects the substantial intranight variability of OSA and the necessity of our dynamic modeling approach to find dose-dependent metabolic responses to OSA.
Morning cardiometabolic outcomes and hemodynamics
As shown in Table 3, OSA increased morning augmentation index (P = 0.008), evening systolic BP (P = 0.017), and nonsignificantly increased morning systolic BP (P = 0.161), and reduced RHI (P = 0.084). Otherwise, OSA did not affect morning OGTT parameters, morning cholesterol levels, or hsCRP. However, we were able to detect patterned responses to OSA by correlating average changes in metabolism, vascular function, and sleep apnea severity between nights (Supplemental Table 6 (212KB, docx) ). For example, clustering occurred in changes of (1) nocturnal FFA and TG; (2) glucose, insulin, cortisol, and HR; and (3) low-density lipoprotein (LDL) cholesterol, hsCRP, glucose, and vascular function.
Table 3.
Hemodynamics and Morning Outcomes
|
Parameter |
CPAP |
OSA |
P |
|---|---|---|---|
| Hemodynamics | |||
| RHI | 2.13 ± 0.119 | 1.99 ± 0.12 | 0.084 |
| Augmentation index, % | 1.39 ± 3.54 | 5.34 ± 3.46 | 0.008 |
| Systolic BP, evening, mm Hg | 123 ± 1.54 | 128 ± 2.12 | 0.017 |
| Diastolic BP, evening, mmHg | 76.6 ± 1.5 | 77.4 ± 1.5 | 0.681 |
| Systolic BP, morning, mmHg | 126 ± 3.02 | 129 ± 3.27 | 0.161 |
| Diastolic BP, morning, mmHg | 75.8 ± 1.78 | 76.5 ± 2.02 | 0.579 |
| Morning lipids and inflammation | |||
| TGs, mg/dL | 107 ± 11.9 | 106 ± 11.1 | 0.934 |
| Total cholesterol, mg/dL | 143 ± 7 | 147 ± 6.83 | 0.356 |
| LDL-C, mg/dL | 89.6 ± 5.71 | 91.1 ± 5.52 | 0.683 |
| HDL-C, mg/dL | 33.4 ± 1.95 | 34.9 ± 2.32 | 0.243 |
| hsCRP, mg/L | 5.13 ± 0.922 | 5.92 ± 1.18 | 0.190 |
| Morning glucose tolerance | |||
| OGTT (AUC glucose) | 19,987 ± 1364 | 21,069 ± 1447 | 0.177 |
| OGTT (AUC insulin) | 11,716 ± 1136 | 12,582 ± 1496 | 0.503 |
Values are shown as mean ± standard error.
Abbreviations: AUC, area under the curve; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Endogenous glucose kinetics
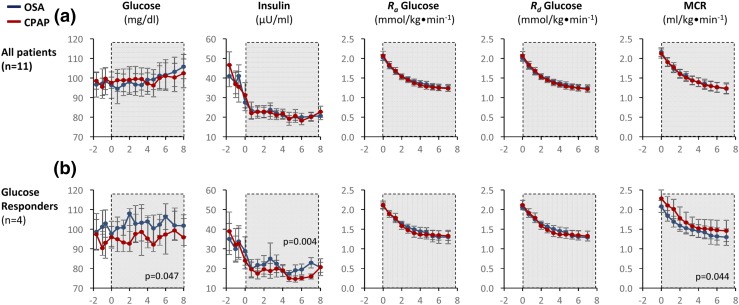
Eleven patients (five men, six women) without diabetes received a primed continuous infusion of 6,6-[2H2] glucose. Irrespective of OSA/CPAP status, glucose Ra gradually decreased ∼25% during sleep, as reported previously by Clore et al. (16). OSA did not affect glucose, insulin, glucose Ra, Rd, or MCR (Fig. 3). In “responder” patients who exhibited a ≥10% increase in glucose during CPAP withdrawal (n = 4), Ra glucose did not change, implicating a fall in glucose clearance (MCR, P = 0.044). The concomitant and early rise in plasma insulin (P = 0.004) in these patients suggested peripheral insulin resistance.
Figure 3.
Effect of CPAP withdrawal on glucose kinetics in patients receiving 6,6-[2H2] glucose. (a) Plasma glucose, insulin, rate of glucose appearance (Ra), disappearance (Rd), or MCR were unchanged (n = 11). (b) In patients who exhibited OSA-induced hyperglycemia (n = 4), there was no increase in glucose production but a fall in MCR and an early and persistent rise in insulin, suggesting peripheral insulin resistance.
Discussion
In this study, we examined the impact of OSA elicited by acute CPAP withdrawal, on the frequently sampled metabolic profile during sleep. Our main finding was that OSA dynamically increased plasma FFA, glucose, and cortisol in a manner that paralleled the distribution of respiratory events, hypoxemia, HR accelerations, and sleep fragmentation during sleep. Many metabolic parameters normalized shortly after awakening in the morning. Additionally, amongst nondiabetic patients, we found that OSA did not increase glucose production but increased plasma insulin and reduced glucose clearance. Finally, we demonstrated that OSA recurrence after a 3-day CPAP hiatus induced cardiovascular stress, including increased BP, HR, and arterial stiffness. In the following discussion, we place these findings in context and address the potential mechanisms and clinical implications.
Lipid metabolism
Previously, we showed that sleep apnea in heart failure patients increases plasma FFA, and the increase was attenuated by supplemental oxygen (17). We now expand on this finding by showing that plasma FFA increased during sleep in a larger group of OSA patients without heart failure; that FFA increased as a function of AHI, hypoxemia, or HR; and that CPAP mitigated lipolysis. Elevated plasma FFA can lead to ectopic lipid deposition and insulin resistance, fatty liver, dyslipidemia, and endothelial dysfunction (18). Nocturnal FFA elevation preceded the onset of daytime insulin resistance during chronic high-fat feeding in dogs (19). Reesterification of fatty acids in the liver and subsequent secretion in very-LDL particles may explain the association we observed between increased nocturnal FFA and TG levels. Similarly, FFA elevations may account for the increased prevalence of diabetes, dyslipidemia, fatty liver disease, and endothelial dysfunction in OSA patients (20–22). In this study, three nights of CPAP withdrawal may not have been sufficient to increase plasma cholesterol levels. Patients were also taking statins and metformin, which can confound relationships between FFA and cholesterol levels.
Glucose metabolism
CPAP withdrawal increased nocturnal glucose by ∼6 mg/dL. This effect was primarily, though not exclusively, driven by diabetic patients, in whom nocturnal glucose increased by ∼17 mg/dL and who comprised many of the glucose responders. Several studies have addressed impacts of OSA on glucose metabolism, with mixed results (1, 23). Apparently, the presence of established (24) or prediabetes (25) and optimal CPAP adherence are required to observe the full extent of OSA-related hyperglycemia. Insulin resistance and beta cell dysfunction likely constitute “loading factors” predisposing to stress hyperglycemia. Diabetes could also be a consequence of recurring exposure to OSA in robust responders. To understand mechanisms of OSA-induced hyperglycemia, we assessed plasma glucose turnover during sleep by stable isotope dilution. Insulin-glucose clamp studies have demonstrated insulin resistance during wakefulness in OSA patients (1). However, the clamp technique was not designed to evaluate effects of an exogenous stimulus such as OSA on glucose kinetics. Using a tracer that does not interfere with endogenous glucose homeostasis, we determined that overnight glucose production was strongly affected by sleep-wake state but not by OSA. Thus, reduced glucose clearance caused hyperglycemia during CPAP withdrawal, at least among nondiabetics of this study. The morning OGTT was not affected, which could be due to rapid improvement in glucose homeostasis upon awakening, or intact compensatory insulin secretion. Nocturnal insulin levels increased in glucose responders, suggesting the latter scenario.
Cardiovascular physiology and inflammation
BP and augmentation index increased and RHI decreased (trend) following CPAP withdrawal, signifying arterial stiffness and impaired endothelial function. Longitudinal OSA studies reported similar outcomes (26, 27). Rapid emergence of cardiovascular stress after acute CPAP withdrawal underscores the importance of adherence to therapy. CPAP did not decrease rates of CVD in recent trials, but CPAP adherence was poor (28, 29). Furthermore, most patients in these studies were taking beta-blockers, which might mitigate responses to OSA, as described later. In terms of inflammation, we did not observe a change in hsCRP after CPAP withdrawal. This suggests that systemic inflammation, at least as ascertained by hsCRP, was not a mediator of the metabolic changes we observed. However, some patients exhibited simultaneous increases in hsCRP with glucose and lipids (Supplemental Tables 5 and 6 (212KB, docx) ).
Mechanisms
The constellation of lipolysis, hyperglycemia, insulin resistance, elevated cortisol, BP, and HR during CPAP withdrawal resembles the cardiometabolic response to other stressors (30–32). Stressful stimuli of OSA include intermittent hypoxia and sleep fragmentation, which activate the sympathetic nervous system (SNS) (33) and the hypothalamic–pituitary–adrenal (HPA) axis (34). Healthy humans exposed to intermittent hypoxia (35) or sleep fragmentation (36) became glucose intolerant in association with increased SNS activity. Hence, we postulate that SNS and HPA responses to OSA govern changes in FFA and glucose metabolism during sleep. Intermittent hypoxia may directly induce oxidative stress and inflammation in tissues (37). However, lactate levels during CPAP withdrawal were not indicative of oxygen insufficiency, and their increase with HR and reduction with sleep suggests catecholamine-stimulated aerobic glycolysis (38). Moreover, in mice exposed to intermittent hypoxia, SNS blockade or carotid body denervation prevented FFA and glucose elevations (4, 6), implicating arterial chemoreflexes.
Clinical significance
We identified a form of sleep-induced metabolic syndrome in OSA patients. Patients who consistently mount exuberant metabolic responses to OSA may be at risk for diabetes and CVD. These might also be patients with a hyperactive response to stress in general, who are susceptible to cardiometabolic disease for reasons other than OSA. Importantly, our findings challenge exclusive reliance on AHI for OSA risk stratification. We found that nocturnal HR, hypoxemia, sleep fragmentation, and diabetes status were more informative for predicting real-time metabolic outcomes. In effect, OSA may be regarded as a “metabolic stress test,” where (1) an individual’s reflexive responses to obstructed breathing and (2) that individual’s baseline metabolic fitness interactively govern the risk of cardiometabolic disease. The marked OSA-induced hyperglycemia we observed in patients with diabetes highlights the importance of diagnosing and treating OSA in such patients. Going forward, metrics we examined (particularly HR) should be validated in other cohorts as a predictor of morbidity and mortality. It is also critical to determine whether daytime sleepiness is associated with nocturnal metabolic dysfunction, as this dictates the need to screen or treat asymptomatic OSA patients. Lastly, we identify SNS activation as a pharmacological target to mitigate metabolic sequelae of OSA.
Limitations
Our study should be interpreted with several caveats. First, we studied CPAP-adherent patients with severe OSA, which limits generalizability of our findings. Second, we included patients with diabetes and morbid obesity. We will require a larger and less complex cohort to examine determinants of OSA responses in a more comprehensive manner. Third, although we standardized the meal before sleep, we did not control diet or activity prior to the study. Fourth, we did not use a “sham CPAP” (subtherapeutic CPAP) control, because research subjects can usually tell they are on sham CPAP if they have previously experienced therapeutic CPAP (39). Therefore, we cannot exclude a placebo effect, such as anxiety about discontinuing CPAP, on nocturnal metabolism. Fifth, acute CPAP withdrawal may not reflect the natural history of chronically untreated OSA. However, we previously showed increases in FFA in treatment-naïve patients (3), and others showed hyperglycemia that improved after initiating CPAP (25). Finally, we administered glucose tracer to nondiabetics only, to avoid confounding effects of insulin resistance and hypoglycemic drugs. Thus, we cannot draw conclusions about nocturnal glucose production during sleep in patients with diabetes with OSA.
In conclusion, OSA increases overnight FFA and glucose in association with SNS and HPA activation. Clinicians should be aware of the vulnerability of OSA patients to metabolic dysfunction during sleep and the cardiovascular effects of even short-term OSA exposure. Diabetic patients are particularly susceptible to nocturnal glucose elevation. Further studies are needed to establish determinants, mechanisms, and long-term significance of these responses.
Acknowledgments
Acknowledgments
This study was funded by the Mid-Atlantic Nutrition Obesity Research Center under National Institute of Diabetes and Digestive and Kidney Diseases Grant P30DK072488; National Heart, Lung, and Blood Institute Grant 1K08HL109475; National Institutes of Health Grants R01HL133100 and R01HL128970; and an American Academy of Sleep Medicine Foundation Junior Faculty Award 106-JF-14.
Clinical trial registry: ClinicalTrials.gov no. NCT02824263 (registered 18 March 2016).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHI
- apnea-hypopnea index
- BP
- blood pressure
- CPAP
- continuous positive airway pressure
- CVD
- cardiovascular disease
- FFA
- free fatty acid
- HR
- heart rate
- HPA
- hypothalamic-pituitary-adrenal
- hsCRP
- high-sensitivity C-reactive protein
- LDL
- low-density lipoprotein
- MCR
- metabolic clearance rate
- OGTT
- oral glucose tolerance test
- OSA
- obstructive sleep apnea
- PSG
- polysomnography
- RHI
- reactive hyperemia index
- SNS
- sympathetic nervous system
- SpO2
- oxygen saturation
- tcCO2
- transcutaneous CO2
- TG
- triglyceride.
References
- 1.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol. 2012;3:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonsignore MR, Marrone O, Insalaco G, Bonsignore G. The cardiovascular effects of obstructive sleep apnoeas: analysis of pathogenic mechanisms. Eur Respir J. 1994;7(4):786–805. [DOI] [PubMed] [Google Scholar]
- 3.Alzoghaibi MA, Bahammam AS. The effect of one night of continuous positive airway pressure therapy on oxidative stress and antioxidant defense in hypertensive patients with severe obstructive sleep apnea. Sleep Breath. 2012;16:499–504. [DOI] [PubMed] [Google Scholar]
- 4.Jun JC, Shin MK, Devera R, Yao Q, Mesarwi O, Bevans-Fonti S, Polotsky VY. Intermittent hypoxia-induced glucose intolerance is abolished by alpha-adrenergic blockade or adrenal medullectomy. Am J Physiol Endocrinol Metab. 2014;307(11):E1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin MK, Han W, Bevans-Fonti S, Jun JC, Punjabi NM, Polotsky VY. The effect of adrenal medullectomy on metabolic responses to chronic intermittent hypoxia. Respir Physiol Neurobiol. 2014;203:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin MK, Yao Q, Jun JC, Bevans-Fonti S, Yoo DY, Han W, Mesarwi O, Richardson R, Fu YY, Pasricha PJ, Schwartz AR, Shirahata M, Polotsky VY. Carotid body denervation prevents fasting hyperglycemia during chronic intermittent hypoxia. J Appl Physiol. 2014;117(7):765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newhouse LP, Joyner MJ, Curry TB, Laurenti MC, Man CD, Cobelli C, Vella A, Limberg JK. Three hours of intermittent hypoxia increases circulating glucose levels in healthy adults. Physiol Rep. 2017;5(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahat B, Chassé É, Mauger JF, Imbeault P. Effects of acute hypoxia on human adipose tissue lipoprotein lipase activity and lipolysis. J Transl Med. 2016;14(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, Stradling JR. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184(10):1192–1199. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun JC, Unnikrishnan D, Schneider H, Kirkness J, Schwartz AR, Smith PL, Polotsky VY. Effect of acute intermittent CPAP depressurization during sleep in obese patients. PLoS One. 2016;11(1):e0146606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bier DM, Leake RD, Haymond MW, Arnold KJ, Gruenke LD, Sperling MA, Kipnis DM. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977;26(11):1016–1023. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe RR, Chinkes DL. Isotopic Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2nd ed. New York, NY: Wiley; 2004. [Google Scholar]
- 14.Albert PS. Longitudinal data analysis (repeated measures) in clinical trials. Stat Med. 1999;18(13):1707–1732. [DOI] [PubMed] [Google Scholar]
- 15.Healey CJ, Fedullo AJ, Swinburne AJ, Wahl GW. Comparison of noninvasive measurements of carbon dioxide tension during withdrawal from mechanical ventilation. Crit Care Med. 1987;15(8):764–768. [DOI] [PubMed] [Google Scholar]
- 16.Clore JN, Nestler JE, Blackard WG. Sleep-associated fall in glucose disposal and hepatic glucose output in normal humans: putative signaling mechanism linking peripheral and hepatic events. Diabetes. 1989;38(3):285–290. [DOI] [PubMed] [Google Scholar]
- 17.Jun JC, Drager LF, Najjar SS, Gottlieb SS, Brown CD, Smith PL, Schwartz AR, Polotsky VY. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep. 2011;34(9):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14(3):281–287. [DOI] [PubMed] [Google Scholar]
- 19.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292(6):E1590–E1598. [DOI] [PubMed] [Google Scholar]
- 20.Jun J, Polotsky VY. Metabolic consequences of sleep-disordered breathing. ILAR J. 2009;50(3):289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drager LF, Jun J, Polotsky VY. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: A systematic review and meta-analysis. Respirology. 2015;20(6):889–895. [DOI] [PubMed] [Google Scholar]
- 23.Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Launois S, Borel AL, Levy P, Pepin JL. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: a systematic review from sham CPAP randomized controlled trials. Sleep Med Rev. 2015;21:23–38. [DOI] [PubMed] [Google Scholar]
- 24.Mokhlesi B, Grimaldi D, Beccuti G, Van Cauter E. Effect of one week of CPAP treatment of obstructive sleep apnoea on 24-hour profiles of glucose, insulin and counter-regulatory hormones in type 2 diabetes. Diabetes Obes Metab. 2017;19(3):452–456. [DOI] [PubMed] [Google Scholar]
- 25.Pamidi S, Wroblewski K, Stepien M, Sharif-Sidi K, Kilkus J, Whitmore H, Tasali E. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lurie A. Hemodynamic and autonomic changes in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:171–195. [DOI] [PubMed] [Google Scholar]
- 27.Lin X, Chen G, Qi J, Chen X, Zhao J, Lin Q. Effect of continuous positive airway pressure on arterial stiffness in patients with obstructive sleep apnea and hypertension: a meta-analysis. Eur Arch Otorhinolaryngol. 2016;273:4081–4088. [DOI] [PubMed] [Google Scholar]
- 28.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS; SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. [DOI] [PubMed] [Google Scholar]
- 29.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. [DOI] [PubMed] [Google Scholar]
- 30.Mook S, Halkes Cj Cj, Bilecen S, Cabezas MC. In vivo regulation of plasma free fatty acids in insulin resistance. Metabolism. 2004;53(9):1197–1201. [DOI] [PubMed] [Google Scholar]
- 31.Stoney CM, Matthews KA, McDonald RH, Johnson CA. Sex differences in lipid, lipoprotein, cardiovascular, and neuroendocrine responses to acute stress. Psychophysiology. 1988;25(6):645–656. [DOI] [PubMed] [Google Scholar]
- 32.Moberg E, Kollind M, Lins PE, Adamson U. Acute mental stress impairs insulin sensitivity in IDDM patients. Diabetologia. 1994;37(3):247–251. [DOI] [PubMed] [Google Scholar]
- 33.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kritikou I, Basta M, Vgontzas AN, Pejovic S, Fernandez-Mendoza J, Liao D, Bixler EO, Gaines J, Chrousos GP. Sleep apnoea and the hypothalamic-pituitary-adrenal axis in men and women: effects of continuous positive airway pressure. Eur Respir J. 2016;47(2):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106(5):1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mesarwi OA, Sharma EV, Jun JC, Polotsky VY. Metabolic dysfunction in obstructive sleep apnea: a critical examination of underlying mechanisms. Sleep Biol Rhythms. 2015;13(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999;354(9177):505–508. [DOI] [PubMed] [Google Scholar]
- 39.Djavadkhani Y, Marshall NS, D’Rozario AL, Crawford MR, Yee BJ, Grunstein RR, Phillips CL. Ethics, consent and blinding: lessons from a placebo/sham controlled CPAP crossover trial. Thorax. 2015;70(3):265–269. [DOI] [PubMed] [Google Scholar]