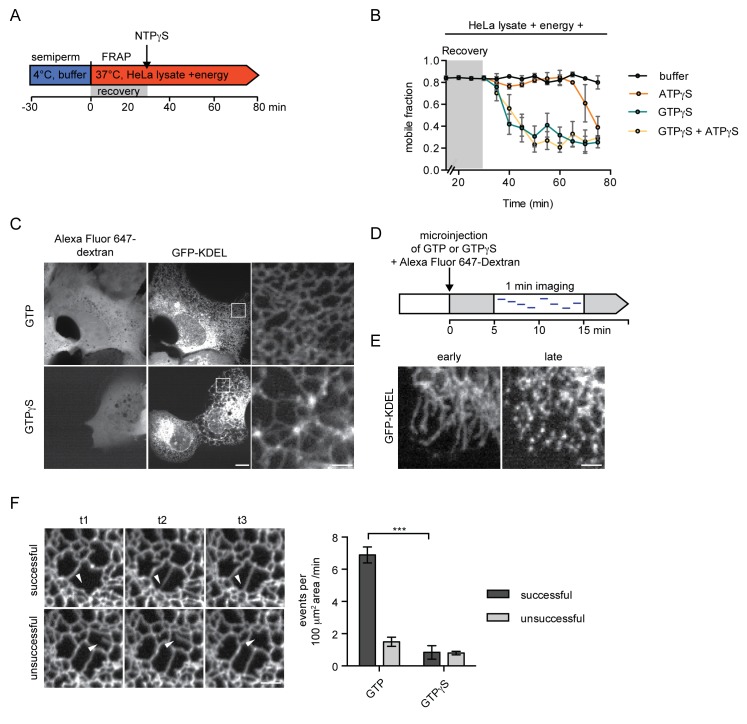
Figure 3. Inhibition of GTP hydrolysis affects ER morphology.
(A) Schematic of experimental set-up in B. (B) 2xRFP-tev-LBR(1-245)-GFP expressing reporter cells were semi-permeabilized and allowed to recover in HeLa cell lysate and energy at 37°C. After 30 min, non-hydrolyzable ATP or GTP analogs were added to a final concentration of 0.3 mM. FRAP was performed every 5 min, starting during the recovery phase and continued after the addition of the nucleotide analogs. Mobile fractions over time. Mean ±SEM, N ≥ 3; n ≥ 20. (C) Morphology of the peripheral ER network of U2OS cells expressing GFP-KDEL after microinjection of either GTP or GTPγS. Alexa Fluor 647-labeled dextran served as injection marker. Scale bars, 10 μm, 2 μm for zoomed panels. (D) Schematic representation of the experiment in E and F. (E) U2OS cells expressing EGFP-KDEL were microinjected with a solution containing 10 mM GTP or GTPγS and a fluorescent dextran. 5 to 15 min after injection, cells were imaged over a time course of 1 min (1 frame/s) to visualize membrane dynamics in the peripheral ER network. Representative images of cells microinjected with GTPγS. Cells imaged early (after ~5 min) after microinjection displayed long unconnected tubules, cells imaged later (after ~15 min) showed fragmentation of the peripheral ER. Scale bar, 2 μm. (F) ER dynamics in the movies of (E) was quantified by scoring the number of successful (example in top panel) or unsuccessful membrane tubule attachments (example in bottom panel) in a 100 μm2 area of cells injected with either GTP or GTPγS (Videos 1 and 2). Mean ±SEM; N ≥ 3; n ≥ 13; two 100 μm2 squares for each cell; ***p<0.0005. Scale bar, 2 μm.