Fig. 2.
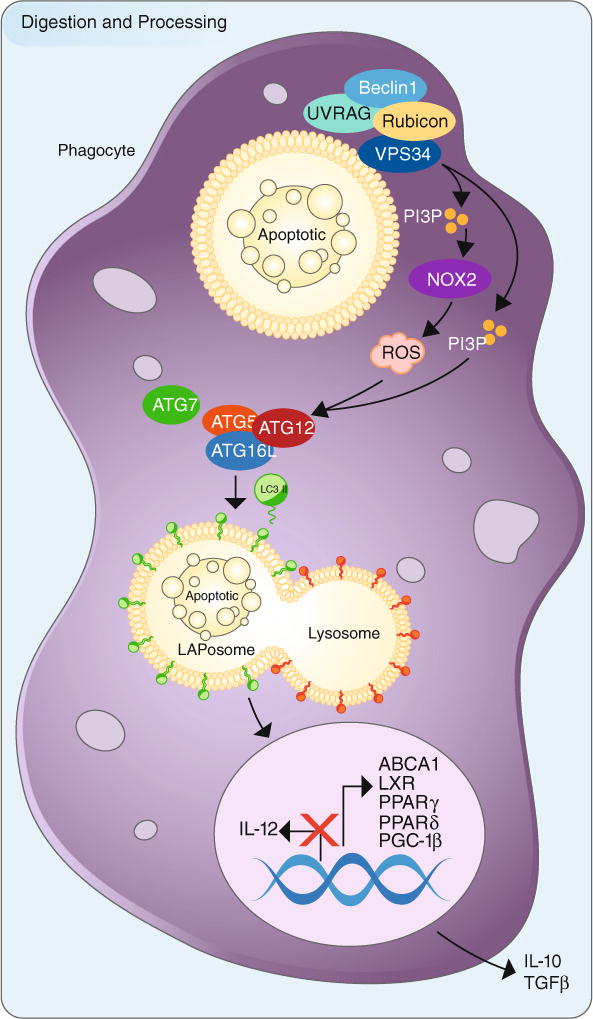
The processing and digestion of engulfed apoptotic cells utilizes LC3-associated phagocytosis and promotes an anti-inflammatory response. Upon engulfment of apoptotic cells (or other dying cells), components of the LC3-associated phagocytosis (LAP) pathway are recruitment to dead cell-containing phagosome (or LAPosome). The Class III PI3 K complex, composed of Beclin-1, VPS34, UVRAG, and Rubicon, is critical to the sustained and localized production of PI(3)P at the LAPosome. PI(3)P serves two roles—the recruitment of the downstream autophagic/LAP machinery (such as ATG5, ATG12, ATG16L, ATG7) and stabilization of the NOX2 complex for the production of ROS. Of note, Rubicon is also required for the stabilization of the NOX2 complex. Both ROS and PI(3)P are required for lipidation and translocation of LC3-II to the single membrane of the LAPosome, and LC3-II is required for fusion to the lysosome and maturation of LAPosome. The anti-inflammatory effects of efferocytosis are mediated by the activity of lipid and cholesterol sensors, such as ABCA1, LXR, PPARγ, PPARδ, and PGC-1β, leading to the production of anti-inflammatory mediators, IL-10 and TGFβ. Pro-inflammatory mediators, such as IL-12, are actively repressed. Perturbations in this process can result in inflammation and autoimmunity
