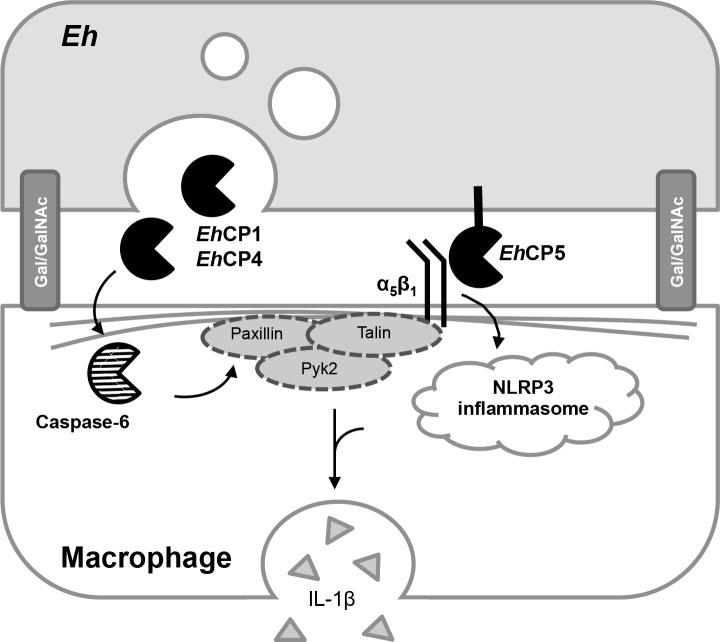
Fig 9. Schematic representation of the Eh-macrophage interacting proteins at the intercellular junction.
Contact between Eh and macrophages are initiated by high affinity binding with the Gal-lectin. EhCP-A5, which is expressed at the surface of Eh, has been shown to bind to the α5β1 integrin at the surface of macrophages through the RGD motif. This in turn activates the NLRP3 inflammasome and triggers IL-1β secretion by macrophages. In parallel, EhCP-A1 and EhCP-A4, which are localized in intracellular vesicles, are released at the intercellular junction. Through an unidentified mechanism, these cysteine proteinases trigger caspase-6 activation and subsequent cleavage of cytoskeletal-associated proteins paxillin, Pyk2 and talin. This arm of the Eh-macrophage interaction contributes to enhance IL-1β release.