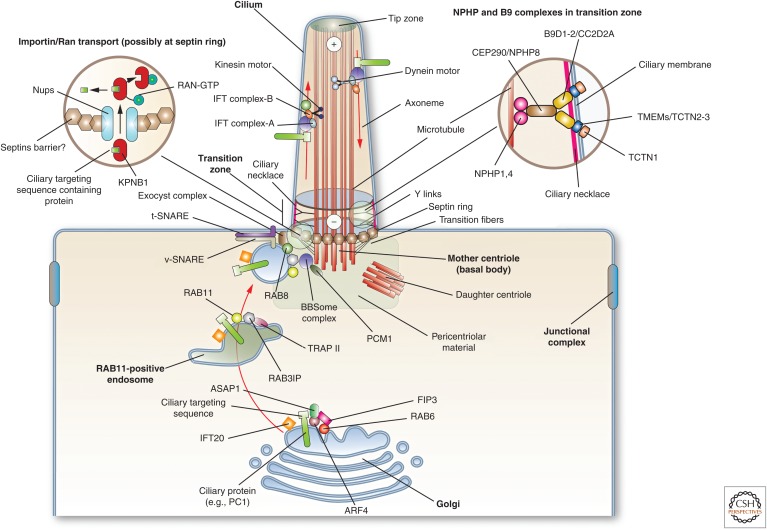
Figure 3.
Mechanisms of protein transport into cilia. At the Golgi, cilia resident proteins are recognized and packaged for delivery. In the case of PC1, its ciliary targeting sequence is recognized by ARF4, and is then delivered to RAB11-positive endosomes by way of a RAB6-, ARF4-, ASAP1-, and FIP3-dependent mechanism. The only intraflagellar transport (IFT) protein localized to the Golgi, IFT20, may have a role in promoting protein delivery to the cilium. At RAB11-positive endosomes, RAB11 recruits the TRAPII complex and the RAB8 GEF RAB3IP. The latter interacts with the basal body–localized BBSome complex (through the BBS1 subunit), and also recruits and activates RAB8. The BBSome is recruited by PCM1. RAB8 along with the exocyst complex and SNAREs, promotes the docking and fusion of the endocytic vesicle at the base of the cilium. Entry into the cilium is regulated at the transition zone by a septin ring, and the Y-links formed by the NPHP and B9 protein complexes. In addition, import into the cilium may depend on a RAN-GTP/GDP gradient (cytoplasmic RAN-GDP is not shown), which regulates KPNB1 binding to ciliary proteins and their subsequent transport across cilia-associated nucleoporins. Once in the axoneme, the ciliary proteins move along the microtubules by interactions with IFT particles and motor proteins. Anterograde traffic (directed toward the tip of the cilium) is mediated by IFT-B and KIF3 motor complex, whereas retrograde is mediated by IFT-A and the dynein-2 motor complex. The BBSome regulates the IFT complexes assembly at the basal body and its turnaround from anterograde to retrograde transport. (Figure from Apodaca and Gallo 2013; adapted, with permission, from the authors.)