Figure 1.
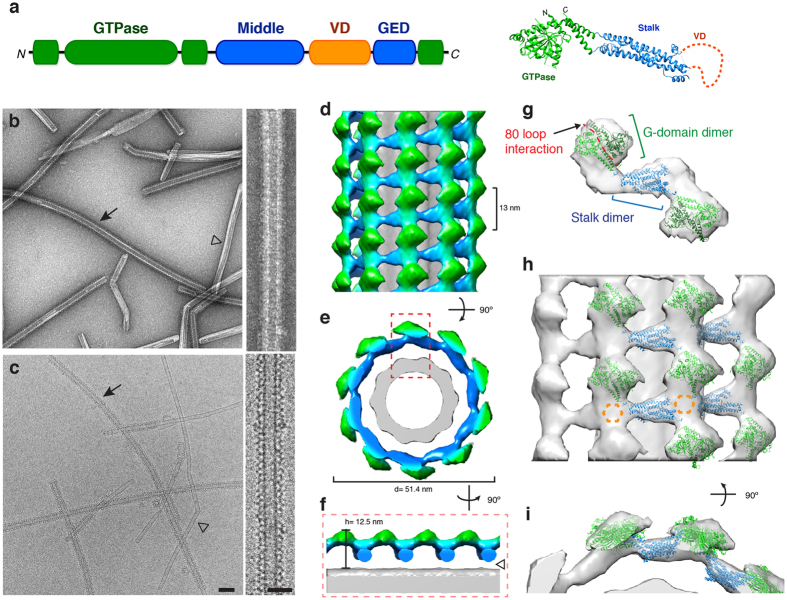
3D structure of Drp1 associated with a phosphatidylserine (PS) lipid template. (a) The primary sequence and tertiary structure (PDB ID: 4BEJ) of Drp1 highlights conserved domains: G domain (green), middle domain (blue), variable domain (orange) and GTPase effector domain (GED, blue). (b–c) Negative stain (b) and cryo-EM (c) images of Drp1 oligomerized in the presence of GMPPCP on galactosyl ceramide (GC) nanotubes containing phosphatidylserine (PS) at low (left; scale bar, 100 nm) and high magnifications (right; scale bar, 50 nm). Filled arrows indicate Drp1 decorated tubes, while open arrowheads indicate undecorated GC/PS tubes. (d–e) The 3D reconstruction of Drp1 on a GC/PS nanotube is presented. The helical pitch (13 nm) and diameter (51.4 nm) are indicated. (f) Cross-section of the 3D structure demonstrates the T-shaped architecture, and a gap between the protein and lipid is highlighted (open arrowhead). (g) The fitted structures of Drp1 GTPase (green) and stalk (blue) dimers are shown. The 80 loop interface (red) mediating G-domain dimerization is highlighted. (h) A side view of multiple Drp1 dimers fitted into the helical density. Unoccupied density is highlighted (dotted orange hexagon). (i) An end-on view of the same fitted structures.