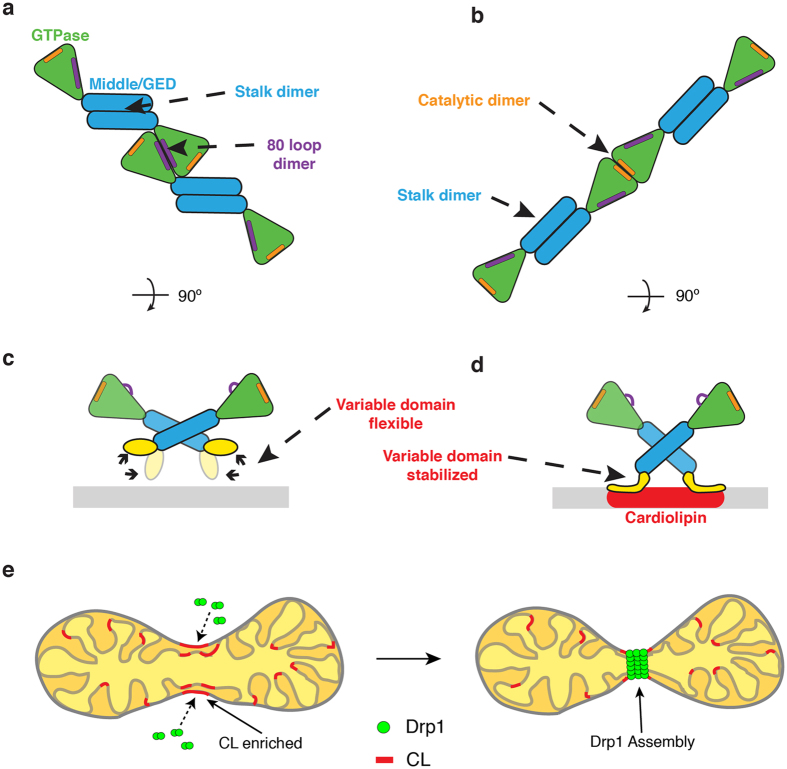
Figure 6.
Cardiolipin interactions trigger active Drp1 assembly. (a) A model illustrates the Drp1 architecture on a GC/PS template. Stalk and 80 loop (purple) G-domain dimerization drive helical assembly. (b) A separate model illustrates the Drp1 architecture on a GC/CL template. Stalk and distinct G-domain dimerizations near the GTP binding pocket (orange) promote assembly of a more active polymer in the presence of CL. (c) On a GC/PS template, the variable domain of Drp1 interacts weakly with the membrane surface. (d) On a GC/CL template, the variable domain is stabilized on the membrane surface, which induces a pivot at the stalk interface to transmit a specific lipid signal at the membrane to the peripheral G domains where an activate conformation is formed. (e) A model depicts Drp1 interactions with CL at the surface of mitochondria where this unique lipid activates Drp1 function at defined sites primed for mitochondrial fission.