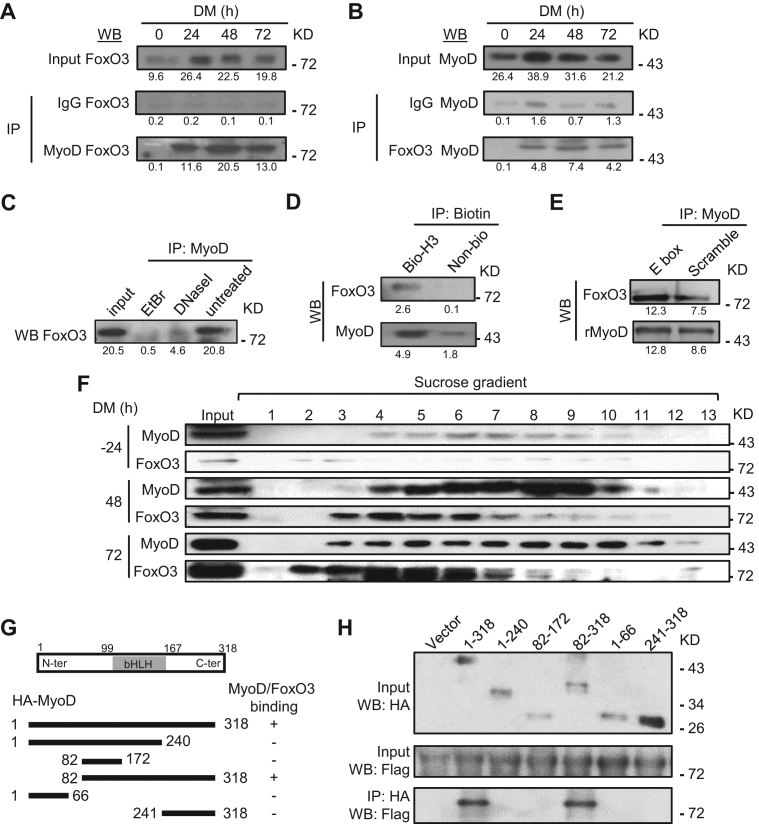
Figure 4.
Physical association of MyoD and FoxO3 at the hotspots in the differentiating MTs. (A) C2C12 was harvested in DM at 0, 24, 48 and 72 h and the lysates were immunoprecipitated with IgG or MyoD antibody and then detected for FoxO3 by western blotting. Numbers below each blot indicate the intensity of each band as measured by Image-Pro Plus. (B) The reciprocal IP was performed with IgG or FoxO3 antibody followed by WB detection of MyoD. (C) The binding between MyoD and FoxO3 at 48 h was abolished by either DNase I or ethidium bromide (EtBr) treatment. (D) When immuniprecipitated by Biotin, biotin-labeled Myogenin hotspot 3 (H3), but not biotin-labeled control, was able to retrieve MyoD and FoxO3. (E) Recombinant MyoD (rMyoD) protein retrieved FoxO3 from MT lysates and the addition of E-box motif DNA increased the retrieval. (F) Sucrose gradient of C2C12 nuclear extracts at DM −24, 48 and 72 h followed by western blotting detected the elution profiles of MyoD and FoxO3, which were found in the same fractions (4–6 and 7) in DM 48 and 72 h. (G and H) The plasmid expressing various domains or the full-length (1-318) of HA-tagged MyoD was co-transfected with plasmid expressing Flag-tagged full length FoxO3. Lysates were pulled down by HA antibody and examined by western blotting using Flag antibody. For all the above panels, the representative images from two replicates are shown.