Abstract
Objective
To evaluate the prescription of potentially inappropriate medications (PIM), using the Screening Tool of Older Persons' potentially inappropriate Prescriptions (STOPP) and Beers criteria, to disabled older people.
Subjects and Methods
One hundred and forty-one patients aged ≥65 years with Barthel scale scores ≤60 and a regular intake of medication for chronic diseases at Chung Shan Medical University Hospital from July to December 2012 were included, and their medical records were reviewed. Comprehensive patient information was extracted from the patients' medical notes. The STOPP and Beers 2012 criteria were used separately to identify PIM, and logistic regression analyses were performed to identify risk factors for PIM. The optimal cutoff for the number of medications prescribed for predicting PIM was estimated using the Youden index.
Results
Of the 141 patients, 94 (66.7%) and 94 (66.7%) had at least one PIM identified by the STOPP and Beers criteria, respectively. In multivariate analysis, PIM identified by the Beers criteria were associated with the prescription of multiple medications (p = 0.013) and the presence of psychiatric diseases (p < 0.001), whereas PIM identified by the STOPP criteria were only associated with the prescription of multiple medications (p = 0.008). The optimal cutoff for the number of medications prescribed for predicting PIM by using the STOPP or Beers criteria was 6. After adjustment for covariates, patients prescribed ≥6 medications had a significantly higher risk of PIM, identified using the STOPP or Beers criteria, compared to patients prescribed <6 medications (both p < 0.05).
Conclusion
This study revealed a high frequency of PIM in disabled older patients with chronic diseases, particularly those prescribed ≥6 medications.
Key Words: Disabled persons, Chronic disease, Older people, Risk factors, Inappropriate prescription, Polypharmacy
Introduction
The use of potentially inappropriate medications (PIM) is a major issue in pharmaceutical therapy for older people because it can increase the risk of adverse drug events [1,2,3]. Older disabled adults, who often have complex comorbidities that require the prescription of multiple medications, may be highly susceptible to PIM use [4]. Previous studies [4,5,6,7,8,9] on the use of PIM have predominantly focused on older populations receiving ambulatory visits or nursing home or home care, and the majority of these populations have a normal or mild-to-moderate functional dependence, but few studies [4,10] have evaluated PIM use in older adults with a marked dependence in activities of daily living (ADL). Several criteria have been developed to identify PIM use in older populations [11,12,13,14]. The Beers criteria, which were proposed by an expert panel using the Delphi method, were originally published in 1991 and were updated newly in 2012. They are the most widely applied criteria to measure geriatric use of PIM [11,15]. A panel of European experts, also using the Delphi consensus method, recently developed a set of PIM-related criteria for elderly people, i.e. the Screening Tool of Older Persons' potentially inappropriate Prescriptions (STOPP), which has been adopted by the European Union Geriatric Medicine Society [13]. Studies have increasingly used the STOPP criteria for the assessment of PIM in older people [7,8,9]. Therefore, the purpose of this study was to evaluate the frequency of prescription of PIM to older adults with marked dependence in ADL, using the STOPP and updated Beers criteria, and to compare the results obtained using each of the two sets of criteria.
Subjects and Methods
Setting and Sample
This study was conducted at Chung Shan Medical University Hospital, a medical centre with >1,300 beds in central Taiwan. Consecutive mobility-disabled patients aged ≥65 years requiring long-term specialized care (such as replacement of the Foley catheter, the nasogastric tube and the tracheal tube and chronic wound care) with and without other medical services in a community setting or at home, who were visited by health-care professionals and received long-term (≥28 days) prescriptions for chronic diseases between July 2012 and December 2012, were enrolled and their medical records were reviewed. Performance in ADL was measured using the Barthel index, with scores ranging from 0 to 100 and scores ≤60 indicating a marked ADL dependence [16]. This study evaluated 141 older mobility-disabled patients receiving the described services in the study hospital. The hospital's institutional review board approved this study, with qualification for a waiver of informed consent.
Data Collection and Measures
Data, including demographic information, medical history, comorbidities and current medications prescribed, were extracted from patients' notes. The STOPP and Beers 2012 criteria were adopted to determine the use of PIM in the study patients [13,15]. If a patient's condition was stable according to a physician's assessment, a long-term prescription was administrated by the NHI program [17]. If a patient had more than one visit for a refill prescription within the study period, only the first occurrence was used in analyses. The disease severity of each patient was assessed using the Charlson comorbidity index (CCI) [18]. Comorbidities were classified according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) [19].
Statistical Analysis
All analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, N.C., USA) and MedCalc Statistical Software version 13.2.2 (Broekstraat, Mariakerke, Belgium). Student's t tests were performed to compare continuous variables between groups. χ2, McNemar and Fisher exact tests were also applied to compare dichotomous variables between groups. The patients were divided into PIM (at least one PIM) and non-PIM (without any PIM) groups, and the risk factors for PIM were determined using logistic regression with purposeful selection of covariates. The methods of Hanley and McNeil [20] were used to analyse receiver-operating characteristic (ROC) curves and to estimate the areas under the ROC curves (AUC). The optimal cutoff number was calculated using the Youden index [maximum (sensitivity + specificity − 1)] [21]. Sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV) and likelihood ratios were then calculated according to the optimal cutoff value. OR and 95% CI were also estimated. A 2-tailed p < 0.05 was considered statistically significant.
Results
Of the 141 older adults with severe disability, 68 (48.2%) were men and 134 (95.0%) had complete ADL dependence (Barthel scale ≤20). The patients' mean age was 81.5 ± 7.0 years (range 65–97) and their mean CCI score was 4.2 ± 2.1 (range 1–13). The mean number of medicines prescribed and coexisting disorders was 7.5 ± 3.4 and 7.7 ± 3.6, respectively. According to systemic disease categories, the patients' most common comorbidities were cardiovascular disorders (n = 124; 87.9%) followed by neurological system disorders (n = 77; 54.6%). The prevalence of PIM identified using the STOPP and Beers 2012 criteria exhibited nonsignificant differences (94/141, 66.7% vs. 94/141, 66.7%). The PIM patients identified using the Beers criteria had a greater number of PIM incidents than those identified using the STOPP criteria (2.5 ± 1.8 vs. 2.0 ± 1.3; p =0.003). Of the PIM patients, 24 were identified by the STOPP criteria only, 24 were identified by the Beers criteria only and 70 were recognized by both sets of criteria. In the 24 patients identified using the STOPP criteria only, calcium channel blockers (CCB) were the most frequently prescribed PIM in 13 (54.2%) patients. However, in the 24 patients identified using the Beers criteria only, benzodiazepines or nonbenzodiazepine hypnotics were the most commonly prescribed PIM in 7 (29.2%) patients.
Based on the STOPP or Beers criteria, the PIM group had a greater number of drugs prescribed and coexisting disorders, and a higher frequency of psychiatric disorders, than the non-PIM group did (all p < 0.05). According to the Beers criteria, the PIM group had a higher percentage (57/94, 60.6% vs. 20/47, 42.6%) of neurological disorders compared to the non-PIM group (p < 0.05). However, according to the STOPP criteria, the percentages (54/94, 56.8% vs. 23/47, 50.0%) of neurological disorders in the two groups exhibited nonsignificant differences (p =0.339; table 1). According to the STOPP criteria, CCB were the most frequently prescribed PIM (28 incidents in 26 patients). However, according to the Beers criteria, benzodiazepines or nonbenzodiazepine hypnotics (Z hypnotics) were the most commonly prescribed PIM (42 incidents in 27 patients).
Table 1.
Baseline and clinical characteristics of 141 disabled older adults with and without PIM use, as identified using the STOPP and Beers 2012 criteria
| Variable | All patients (n = 141) | STOPP |
Beers 2012 |
||
|---|---|---|---|---|---|
| PIM (n = 94) | non-PIM (n = 47) | PIM (n = 94) | non-PIM (n = 47) | ||
| Instances of PIM per person, n | 1.8 ± 1.3* | 2.5 ± 1.8* | |||
| Age, years | 81.5 ± 7.0 | 81.4 ± 7.3 | 81.7 ± 6.3 | 82.2 ± 7.1 | 80.1 ± 6.6 |
| Male gender | 68 (48.2) | 41 (43.6) | 27 (57.4) | 41 (43.6) | 27 (57.4) |
| CCI (points) | 4.2 ± 2.1 | 4.4 ± 2.3 | 4.0 ± 1.9 | 4.4 ± 2.2 | 3.9 ± 2.0 |
| Barthel score ≤ 20 | 134 (95.0) | 88 (93.6) | 46 (97.9) | 87 (92.6) | 47 (100) |
| Prescribed medications, n | 7.5 ± 3.4 | 8.1 ± 3.7+ | 6.4 ± 2.2+ | 8.1 ± 3.5# | 6.4 ± 3.0# |
| Coexisting disorders, n | 7.7 ± 3.6 | 8.1 ± 3.8+ | 6.8 ± 3.1+ | 8.3 ± 3.7# | 6.4 ± 3.0# |
| Coexisting disordersa | |||||
| Cardiovascular | 124 (87.9) | 80 (85.1) | 44 (93.6) | 82 (87.2) | 42 (89.4) |
| Neurological | 77 (54.6) | 54 (56.8) | 23 (50.0) | 57 (60.6)# | 20 (42.6)# |
| Renal | 76 (53.9) | 52 (55.3) | 24 (51.1) | 50 (53.2) | 26 (55.3) |
| Endocrinological | 74 (52.5) | 52 (55.3) | 22 (46.8) | 51 (54.3) | 23 (48.9) |
| Gastrointestinal | 49 (34.8) | 36 (38.3) | 13 (27.7) | 33 (35.1) | 16 (34.0) |
| Psychiatric | 47 (33.3) | 37 (39.4)+ | 10 (21.3)+ | 42 (44.7)# | 5 (10.6)# |
| Respiratory | 46 (32.6) | 31 (33.0) | 15 (31.9) | 27 (28.7) | 19 (40.4) |
| Musculoskeletal or connective tissue | 38 (27.0) | 24 (25.5) | 14 (29.8) | 29 (30.9) | 9 (19.1) |
| Genitourinary | 32 (22.7) | 21 (22.3) | 11 (23.4) | 23 (24.5) | 9 (19.1) |
| Skin/subcutaneous | 20 (14.2) | 14 (14.9) | 6 (12.8) | 13 (13.8) | 7 (14.9) |
| Malignancies | 10 (7.1) | 6 (6.4) | 4 (8.5) | 6 (6.4) | 4 (8.5) |
Values are presented as numbers (%) unless otherwise stated.
Patients included in more than one category were counted in each.
p < 0.05 for comparing PIM incidents per person according to the two sets of criteria.
p < 0.05 for comparing PIM and non-PIM groups according to the STOPP criteria.
p < 0.05 for comparing PIM and non-PIM groups according to the Beers 2012 criteria.
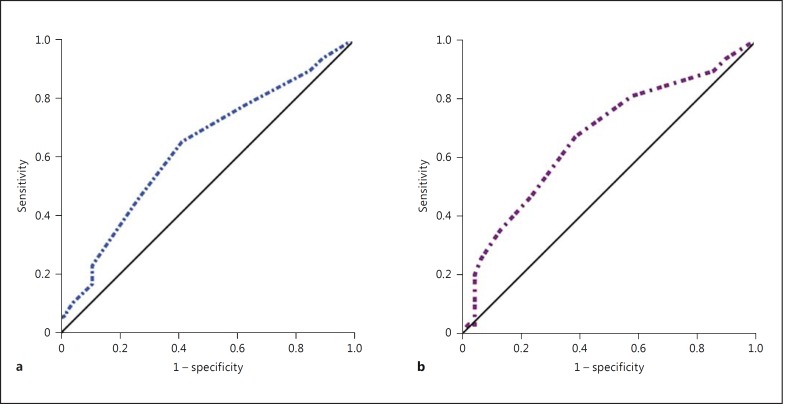
In multivariate analysis, based on the Beers criteria, PIM use was associated with a high number of medicines prescribed (p = 0.013) and the presence of psychiatric disorders (p < 0.001). On the other hand, according to the STOPP criteria, PIM use was only associated with a high number of medicines prescribed (p = 0.008; table 2). The AUC for the number of medications prescribed for predicting the risk of PIM using the STOPP and Beers criteria were 0.644 and 0.673, respectively (both p < 0.05). These values exhibited nonsignificant differences (p = 0.665; fig. 1). According to the Youden index, the optimal cutoff for the number of medications prescribed for predicting PIM using the STOPP or Beers criteria was 6. At a cutoff of ≥6 medications prescribed, the model based on the STOPP criteria had a sensitivity of 79%, a specificity of 38%, a PPV of 72% and an NPV of 47%, whereas the model based on the Beers criteria had a sensitivity of 81%, a specificity of 43%, a PPV of 74% and an NPV of 53%. The logistic regression analyses conducted to compare the risk of PIM in patients with <6 drugs prescribed and in patients with ≥6 drugs prescribed, adjusting for baseline variables (age, sex and CCI) and other risk factors identified using the STOPP or Beers criteria, indicated that patients with ≥6 medications prescribed had a significantly higher risk of PIM, identified using the STOPP or Beers criteria, than patients with <6 medications prescribed (STOPP criteria: adjusted OR = 2.1, 95% CI 1.1–5.0; Beers criteria: adjusted OR = 2.2, 95% CI 1.3–6.6; table 3).
Table 2.
Multivariate analysis of risk factors for PIM use identified using the STOPP or Beers 2012 criteria
| Variables | OR (95% CI) | p value |
|---|---|---|
| According to STOPP criteria | ||
| Number of medications prescribed | 1.21 (1.05–1.38) | 0.008 |
| According to Beers 2012 criteria | ||
| Number of medications prescribed | 1.13 (1.03–1.25) | 0.013 |
| Psychiatric diseases (presence vs. absence) | 5.56 (2.68–11.56) | <0.001 |
Fig. 1.
ROC curves for the number of medications prescribed for predicting the risk of PIM according to the STOPP or Beers 2012 criteria. a When applying the STOPP criteria, the AUC was 0.644 (95% CI 0.559–0.723, p = 0.002. b When applying the Beers 2012 criteria, the AUC was 0.673 (95% CI 0.589–0.749, p < 0.001). The diagonal reference line indicates no discrimination.
Table 3.
Sensitivity, specificity, predictive values, likelihood ratios, and OR for the optimal cutoff for the number of medications prescribed for predicting risk of PIM according to the STOPP and Beers 2012 criteria
| Cutoff ≥6 drugsa | PIM group, n/total (%) | Non-PIM group, n/total (%) | Sen, % (95% CI) | Spe, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | PLR (95% CI) | NLR (95% CI) | Adjusted OR (95% CI) | p |
|---|---|---|---|---|---|---|---|---|---|---|
| STOPP criteria | 74/94 (78.7) | 29/47 (61.7) | 79 (69–87) | 38 (25–54) | 72 (62–80) | 47 (31–64) | 1.3 (1.0–1.6) | 0.6 (0.3–0.9) | 2.1 (1.1–5.0) b | 0.034 |
| Beers 2012 criteria | 76/94 (80.9) | 27/47 (57.4) | 81 (71–88) | 43 (28–58) | 74 (64–82) | 53 (36–69) | 1.4 (1.1–1.8) | 0.5 (0.3–0.8) | 2.2 (1.3–6.6)c | 0.012 |
NLR = Negative likelihood ratio; PLR = positive likelihood ratio; Sen = sensitivity; Spe = specificity.
Optimal cutoff value estimated using the Youden index.
Adjusted for age, sex and severity of comorbidities.
Adjusted for age, sex, severity of comorbidities and history of psychiatric disorders.
Discussion
In this study, the major findings were as follows: (a) two thirds of the patients had been prescribed at least one PIM, irrespectively of screening using the STOPP or Beers 2012 criteria; (b) the prevalence of PIM use identified using the STOPP or Beers 2012 criteria exhibited nonsignificant differences, although the Beers 2012 criteria identified a greater number of PIM incidents in the PIM group compared to the STOPP criteria; (c) according to Beers 2012 criteria the use of PIM was associated with multiple drugs prescribed and the presence of psychiatric diseases, whereas according to the STOPP criteria PIM use was only associated with multiple drugs prescribed, and (d) CCB were the most commonly prescribed PIM identified using the STOPP criteria, whereas benzodiazepine or Z hypnotics were the most commonly prescribed PIM identified using the Beers 2012 criteria.
Our results on the prevalence of PIM in older disabled patients, as identified using the STOPP or Beers 2012 criteria, are higher than the prevalence rates reported in previous studies evaluating elderly people in nursing homes or receiving home care [1,3,4,5]. The differences could have resulted from our patients having an almost complete ADL dependence and multiple complex comorbidities (mean CCI score >4) and thus being weaker physically than the patients in previous reports [1,3,4,5]. Our result that prescription of multiple drugs is a predictor of the risk of PIM is consistent with previous studies' findings [1,3,4,5,8,22]. However, no consensus exists on the minimum number of regular medications that need to be prescribed in order for the use to be considered polypharmacy (it has ranged from 4 to 20 drugs in previous studies) [1,3,4,5,9,22,23,24]. Our data indicated that the number of medications prescribed was predictive of the risk of PIM in older disabled patients, irrespectively of identification using the STOPP or Beers 2012 criteria, and that an optimal cutoff (i.e. ≥6) for the number of drugs prescribed had a modest sensitivity and PPV in the prediction model. Whether the STOPP or Beers 2012 criteria were more sensitive at detecting PIM use in older patients with marked disability was unclear. ROC curve analyses indicated nonsignificant differences in the discriminative power of the two sets of criteria for predicting the risk of PIM, although the Beers 2012 criteria identified a greater number of PIM incidents than the STOPP criteria did. Several studies [1,2,3,5,6,7,8,9,10,11,12,13] have revealed that explicit PIM criteria can be used as health-care quality indicators. However, the STOPP and Beers 2012 sets of criteria are complex (>50 items in each set) and their application in clinical practice is time consuming [2,11,12,13,14,15,24]. It is thus essential to reduce the time required for applying the PIM criteria to increase the practicality of their usage. Electronic applications, such as computerized prescription safety alerts, pharmaceutical decision-making support software, and computerized advice or reminders for prescriptions, have reportedly reduced geriatric PIM in clinical practice [25,26,27,28]. However, these real-time e-prescribing systems are costly and are not obtainable in medical settings with limited resources, particularly in developing countries [26,27].
The high prevalence of benzodiazepine or Z hypnotic usage in patients with PIM identified using the Beers 2012 criteria, but not the STOPP criteria, could have been caused by the Beers 2012 criteria including the use of all benzodiazepines and Z hypnotics, whereas the STOPP criteria considered only the long-term use of long-acting benzodiazepines (or those with long-acting metabolites). However, benzodiazepines (all types) and Z hypnotics can increase the likelihood of cognitive deterioration, delirium, falls, fractures and even death in older people [4,5,11,15,29,30,31]. Hence, we suggest that benzodiazepines or Z hypnotics should be prescribed to older people with caution, and preferably only for short periods when no alternative medications are available. The PIM-related psychoactive drugs that were frequently prescribed to older patients with severe disability, identified using the Beers 2012 criteria, could explain the independent effect of psychiatric disorders on the risk of PIM, as reported previously [5,23,24]. Because the Beers 2012 criteria only listed nondihydropyridine CCB as PIM for older people with chronic constipation or systolic heart failure, CCB became less frequent PIM when using the Beers criteria compared to the STOPP criteria. Constipation could be actuated by dihydropyridine/nondihydropyridine CCB [15,31]; therefore, physicians should consider prescribing medications other than CCB to older people with constipation.
The limitations of this study include its retrospective design and the use of data derived from a single medical center. Although all study patients' prescriptions could be obtained through the Taiwanese NHI program and may minimize the use of over-the-counter drugs, data for over-the-counter medications were not obtained and might thus have underestimated the risk of PIM.
Conclusion
Our data showed that prescription of multiple medications was associated with PIM use, as indicated by the STOPP or Beers 2012 criteria, in older disabled people. According to the Beers 2012 criteria, the presence of psychiatric disorders was also associated with PIM use. Our findings emphasize that physicians should be aware of the high risk of PIM in severely disabled older patients, particularly those taking ≥6 drugs.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Cannon KT, Choi MM, Zuniga MA. Potentially inappropriate medication use in elderly patients receiving home health care: a retrospective data analysis. Am J Geriatr Pharmacother. 2006;4:134–143. doi: 10.1016/j.amjopharm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Cahir C, Fahey T, Teeling M, et al. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69:543–552. doi: 10.1111/j.1365-2125.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao Y, Shao H, Bishop TF, et al. Inappropriate medication in a national sample of US elderly patients receiving home health care. J Gen Intern Med. 2012;27:304–310. doi: 10.1007/s11606-011-1905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiss T, Dreier A, Meinke C, et al. Frequency of inappropriate drugs in primary care: analysis of a sample of immobile patients who received periodic home visits. Age Ageing. 2011;40:66–73. doi: 10.1093/ageing/afq106. [DOI] [PubMed] [Google Scholar]
- 5.Fialová D, Topinková E, Gambassi G, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293:1348–1358. doi: 10.1001/jama.293.11.1348. [DOI] [PubMed] [Google Scholar]
- 6.Barnett K, McCowan C, Evans JMM, et al. Prevalence and outcomes of use of potentially inappropriate medicines in older people: cohort study stratified by residence in nursing home or in the community. BMJ Qual Saf. 2011;20:275–281. doi: 10.1136/bmjqs.2009.039818. [DOI] [PubMed] [Google Scholar]
- 7.Ryan C, O'Mahony D, Kennedy J, et al. Potentially inappropriate prescribing in older residents in Irish nursing homes. Age Ageing. 2013;42:116–120. doi: 10.1093/ageing/afs068. [DOI] [PubMed] [Google Scholar]
- 8.Weng MC, Tsai CF, Sheu KL, et al. The impact of number of drugs prescribed on the risk of potentially inappropriate medication among outpatient older adults with chronic diseases. QJM. 2013;106:1009–1015. doi: 10.1093/qjmed/hct141. [DOI] [PubMed] [Google Scholar]
- 9.Hamano J, Tokuda Y. Inappropriate prescribing among elderly home care patients in Japan: prevalence and risk factors. J Prim Care Community Health. 2014;5:90–96. doi: 10.1177/2150131913518346. [DOI] [PubMed] [Google Scholar]
- 10.Chrischilles E, Rubenstein L, Van Gilder R, et al. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55:29–34. doi: 10.1111/j.1532-5415.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 11.Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 12.Pugh MJ, Hanlon JT, Zeber JE, et al. Assessing potentially inappropriate prescribing in the elderly Veterans Affairs population using the HEDIS 2006 quality measure. J Manag Care Pharm. 2006;12:537–545. doi: 10.18553/jmcp.2006.12.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 14.Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107:543–551. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campanelli CM, American Geriatrics Society 2012 Beers Criteria Update Expert Panel American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wade DT, Collin C. The Barthel ADL index: a standard measure of physical disability? Int Disabil Stud. 1988;10:64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 17.National Health Insurance Administration of the Ministry of Health and Welfare of Taiwan Chronic disease patients (refill prescriptions): special medical needs http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=596&WD_ID=596&webdata_id=3179 (accessed May 27, 2014).
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.International Classification of Diseases, 9th Revision.Clinical Modification (ICD-9-CM) http://www.cdc.gov/nchs/icd/icd9cm.htm (accessed November 10, 2013).
- 20.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 21.Youden WJ. An index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Hu SH, Capezuti E, Foust JB, et al. Medication discrepancy and potentially inappropriate medication in older Chinese-American home-care patients after hospital discharge. Am J Geriatr Pharmacother. 2012;10:284–295. doi: 10.1016/j.amjopharm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Dalby DM, Hirdes JP, Hogan DB, et al. Potentially inappropriate management of depressive symptoms among Ontario home care clients. Int J Geriatr Psychiatry. 2008;23:650–659. doi: 10.1002/gps.1987. [DOI] [PubMed] [Google Scholar]
- 24.Chang CB, Lai HY, Yang SY, et al. Patient- and clinic visit-related factors associated with potentially inappropriate medication use among older home healthcare service recipients. PLoS One. 2014;9:e94350. doi: 10.1371/journal.pone.0094350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon SR, Smith DH, Feldstein AC, et al. Computerized prescribing alerts and group academic detailing to reduce the use of potentially inappropriate medications in older people. J Am Geriatr Soc. 2006;54:963–968. doi: 10.1111/j.1532-5415.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 26.Moxey A, Robertson J, Newby D, et al. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J Am Med Inform Assoc. 2010;17:25–33. doi: 10.1197/jamia.M3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson J, Walkom E, Pearson SA, et al. The impact of pharmacy computerised clinical decision support on prescribing, clinical and patient outcomes: a systematic review of the literature. Int J Pharm Pract. 2010;18:69–87. [PubMed] [Google Scholar]
- 28.Clyne B, Bradley MC, Hughes CM, et al. Addressing potentially inappropriate prescribing in older patients: development and pilot study of an intervention in primary care (the OPTI-SCRIPT study) BMC Health Serv Res. 2013;13:307. doi: 10.1186/1472-6963-13-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61:1128–1134. doi: 10.1111/jgs.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weich S, Pearce HL, Croft P, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ. 2014;348:g1996. doi: 10.1136/bmj.g1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcum ZA, Hanlon JT. Commentary on the new American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults. Am J Geriatr Pharmacother. 2012;10:151–159. doi: 10.1016/j.amjopharm.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]