Figure 4.
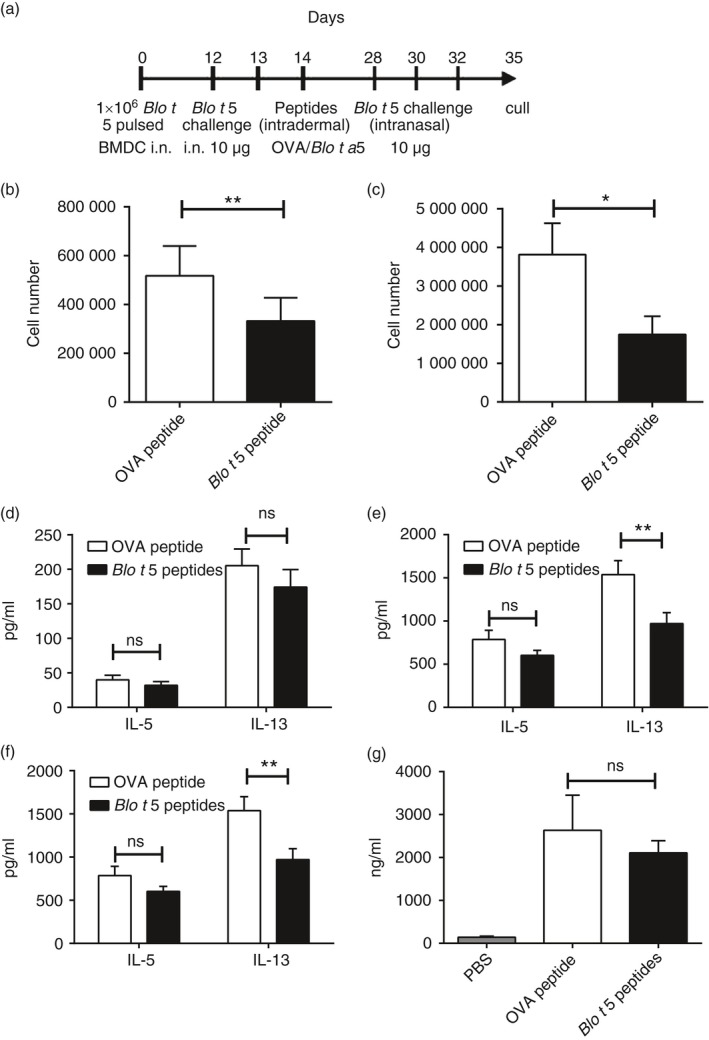
Application of Blo t 5 peptides for peptide immunotherapy of allergic asthma. (a) Experimental protocol for peptide immunotherapy studies. Eosinophilia in the bronchoalveolar lavage (BAL) (b) and lungs (c) were assessed by flow cytometry. Eosinophils were identified by flow cytometry as Siglec‐F+ CD11c– Ly6G– cells (n = 38 and n = 37 for BAL and n = 15 for lung). Interleukin‐5 (IL‐5) and IL‐13 levels in the BAL (d) and lungs (e) were assessed by ELISA. (f) Draining lymph node cells were cultured for 3 days and the cytokine production was assayed by ELISA (n = 18). (g) Total serum IgE levels were not affected by peptide immunotherapy (n = 24). **P < 0·05, **P < 0·01.
