Figure 5.
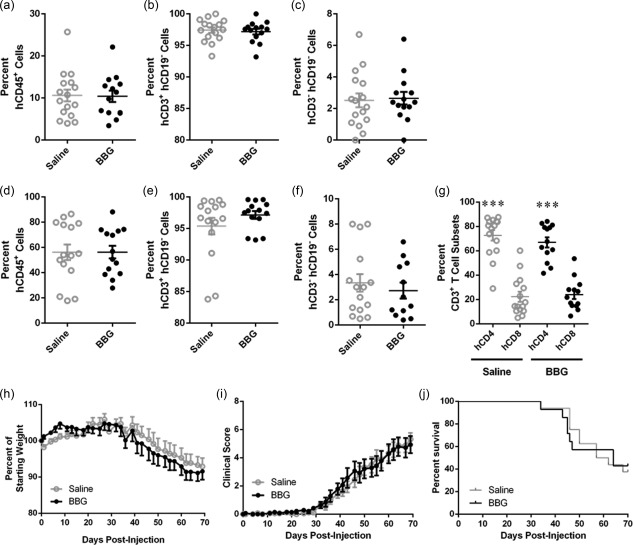
Brilliant Blue G (BBG) does not affect engraftment of human cells. (a–j) Non‐obese diabetic‐severe combined immunodeficiency‐interleukin (NOD‐SCID‐IL)‐2Rγnull (NSG) mice were injected intraperitoneally (i.p.) with 10 × 106 human (h) peripheral blood mononuclear cells (PBMCs), and subsequently with saline (control) (n = 14) or saline containing Brilliant Blue G (BBG) (50 mg/kg) (n = 16) every 2 days (from days 0 to 8), and monitored for clinical signs of graft‐versus‐host disease (GVHD) over 10 weeks. (a–g) The percentage of human leucocytes in (a–c) blood at 3 weeks post‐hPBMC injection and (d–f) spleens at end‐point were determined by flow cytometry. (a,d) hCD45+ leucocytes are expressed as a percentage of total mCD45+ and hCD45+ leucocytes. (b,e) hCD3+hCD19– cells and (c,f) hCD3–hCD19– cells are expressed as a percentage of total hCD45+ leucocytes. (g) hCD4+ and hCD8+ T cell subsets are expressed as a percentage of total hCD3+ leucocytes. Data represent mean ± standard error of the mean (s.e.m.); symbols represent individual mice; ***P < 0·0001 compared to hCD8+ T cells. (h–j) NSG mice were monitored for (a) weight loss, (b) clinical score, and (c) survival. Data represent (h, i) group means ± s.e.m. or (j) percentage survival (saline n = 14, BBG n = 16).
