Figure 6.
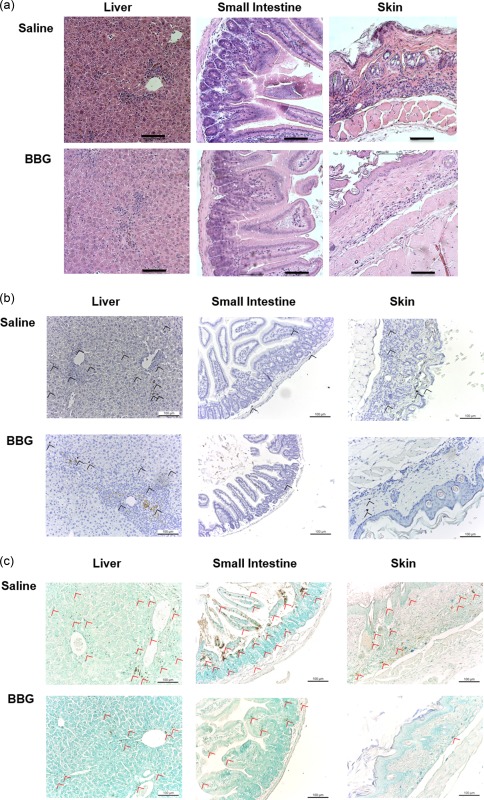
Brilliant Blue G (BBG) does not prevent graft‐versus‐host disease (GVHD) in humanized mice. (a–c) Non‐obese diabetic‐severe combined immunodeficiency‐interleukin (NOD‐SCID‐IL)‐2Rγnull (NSG) mice injected intraperitoneally (i.p.) with 10 × 106 human (h) peripheral blood mononuclear cells (PBMCs) (day 0), and with saline (control) or 50 mg/kg Brilliant Blue G (BBG) (from Fig. 5) were monitored for clinical signs of GVHD over 10 weeks. Tissue sections (liver, small intestine and skin) from hPBMC‐injected mice injected with saline (control) or BBG at end‐point were stained with (a) haematoxylin and eosin, (b) anti‐hCD3 monoclonal antibody with 3,3′‐diaminobenzidine tetrachloride detection system and haematoxylin or (c) terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick‐end labelling with 3,3′‐diaminobenzidine tetrachloride detection system and methyl green. Images were captured by microscopy with each image representative of (a) four or (b,c) two mice per group; bars represent 100 μm; (b) black arrow heads indicate T cells and (c) red arrow heads indicate apoptotic cells.
