Figure 1.
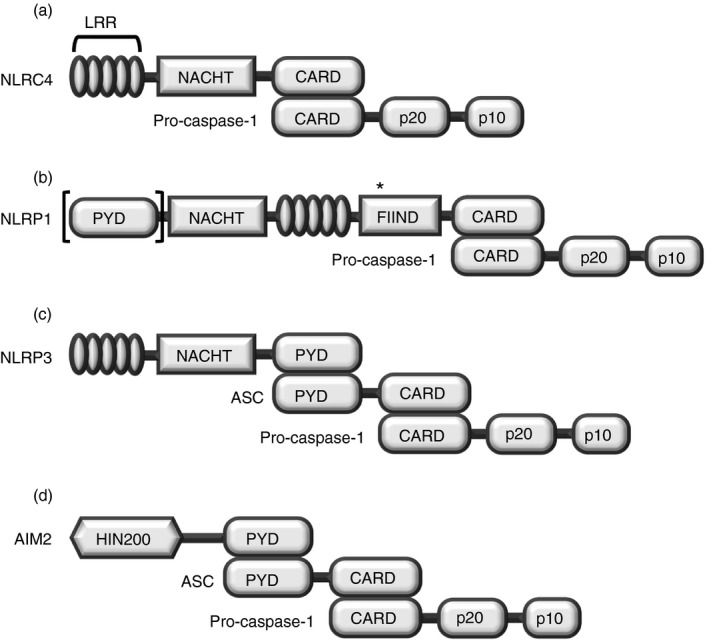
Structures of canonical inflammasomes. (a–c) Three different NLRs, and (d) AIM2 assembly of canonical inflammasomes through binding with pro‐caspase‐1. (a) NLRC4 and (b) NLRP1 (lack of [PYD] in mouse NLRP1 isoforms) assemble canonical inflammasomes by interacting directly with inactive pro‐caspase‐1 through the CARD motif, whereas (c) NLRP3 and (d) AIM2 assemble canonical inflammasomes by interacting indirectly with pro‐caspase‐1 via the bipartite PYD–CARD adaptor protein ASC. After assembly of canonical inflammasomes, pro‐caspase‐1 is cleaved and matures to an active form. Abbreviations used: LRR, leucine‐rich repeat; NRL, nucleotide‐binding oligomerization domain (NOD)‐like receptor; caspase, cysteine‐aspartic protease; CARD, caspase recruit domain; NACHT, nucleotide‐binding and oligomerization domain; FIIND, function to find domain; AIM2, absent in melanoma 2; PYD, pyrin domain; HIN, haematopoietic interferon‐inducible nuclear proteins. *Autocatalytic cleavage.
