Fig. 2. Designed β-sheets and folds.
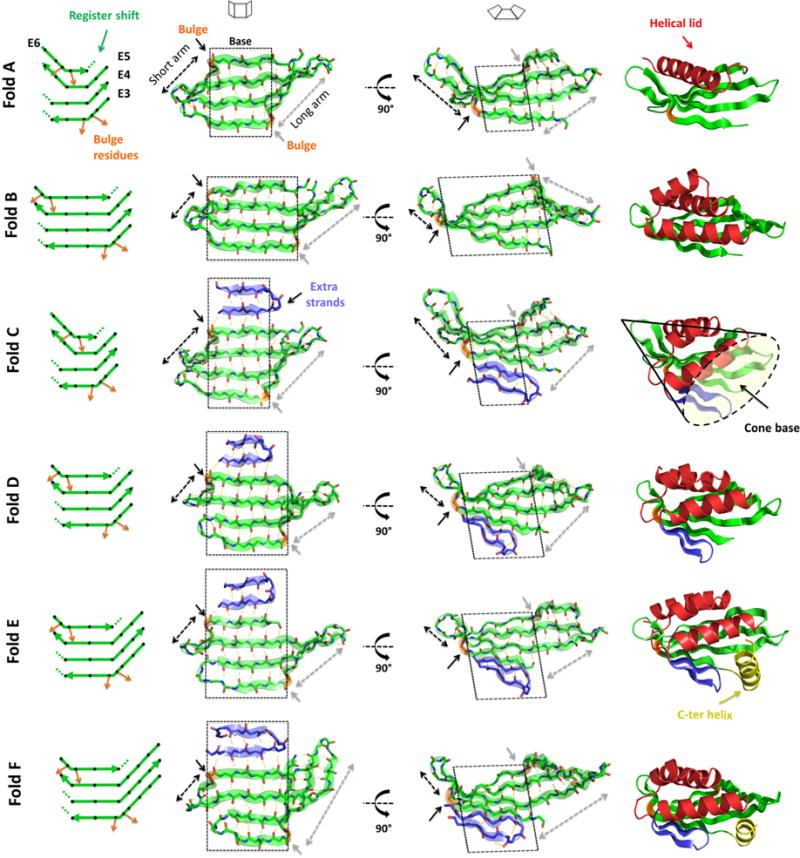
On the left, diagrams of the 4-stranded antiparallel β-sheets. Black diamonds represent residues with sidechains pointing to the convex face of the β-sheet and orange arrows highlight the β-bulge offset in sidechain directionality. Dotted lines show the local termination of strand pairing due to register shift between paired strands. Second and third columns show two views of the designed β-sheets. Black and gray dashed arrows show the length of the short and long arms, respectively, that emerge from the flat central base (highlighted by a black dashed square). On the right, examples of each designed protein fold containing 4-stranded antiparallel β-sheets (green), helical lids (red), extra strands (blue) and a C-terminal helix capping the pocket entrance (yellow). The concave base of these conical folds is well suited for small molecule binding site design.
