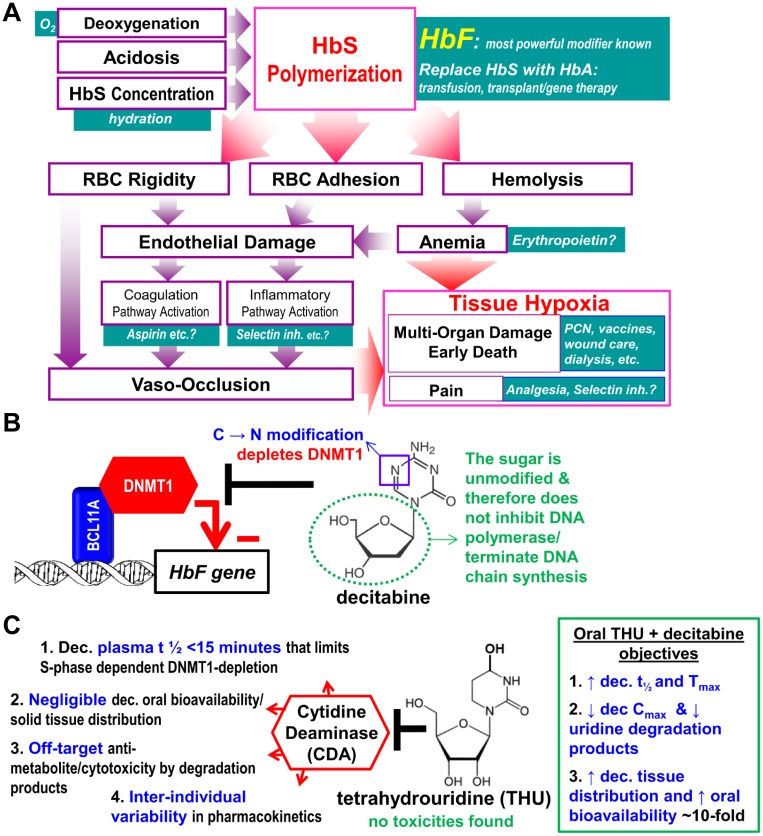
Fig 1. Fetal hemoglobin (HbF) blocks polymerization of deoxy sickle hemoglobin (HbS), the root cause of sickle cell disease (SCD) pathophysiology, and is the most powerful known disease modifier.
(A) Polymerization of deoxy HbS drives all SCD pathophysiology; In contrast to HbF, normal adult hemoglobin (HbA, ẞ-chains) can participate in polymerization. (B) The gene for HbF (HBG) is silenced by DNA methyltransferase 1 (DNMT1). Although DNA-binding factors, e.g., BCL11A, direct this silencing, the biochemical work of epigenetic repression is executed by chromatin-modifying enzymes, amongst which DNMT1 is central. Decitabine depletes DNMT1 and can do so without cytotoxicity because in contrast to other cytidine analogues (e.g., cytarabine) the deoxyribose moiety (green dotted circle) is natural, although higher concentrations do cause anti-metabolite effects and DNA damage, in part by degradation into uridine counterparts that misincorporate into DNA. (C) Several pharmacologic limitations of decitabine hinder safe, effective, practical clinical translation. The limitations have a common cause, the enzyme cytidine deaminase (CDA). Tetrahydrouridine (THU) inhibits CDA. No toxicities have been found for THU in animals or humans.