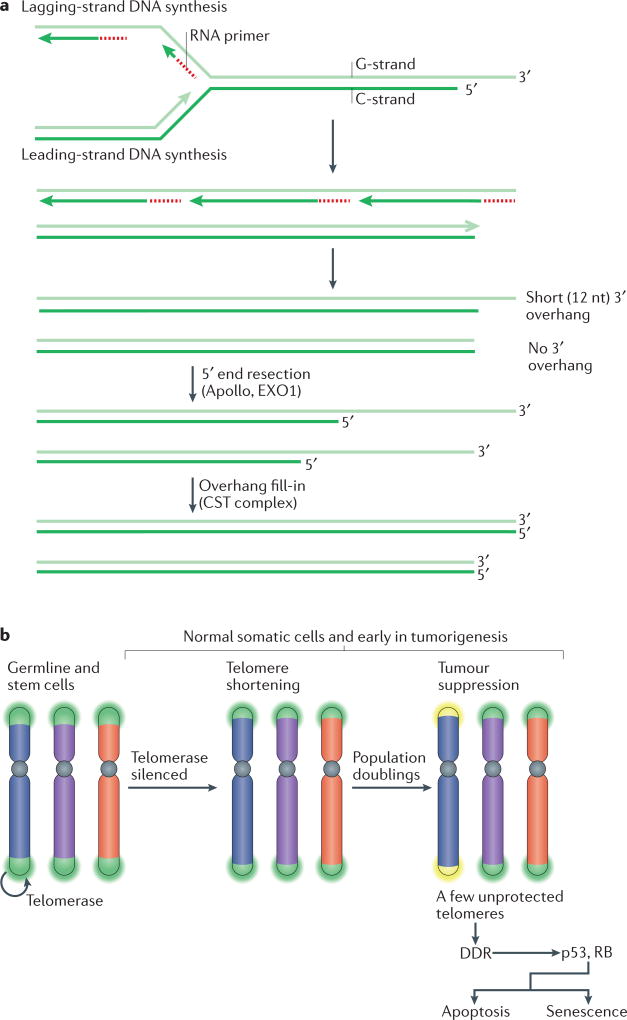
Figure 2. Telomere shortening as a barrier to tumorigenesis.
a | The molecular basis of telomere shortening. Incomplete DNA synthesis at the end of the lagging strand (at the site of the terminal RNA primer) leaves a short 3′ overhang. Additional loss of telomeric DNA occurs through the processing of the leading-strand ends of telomeres to regenerate the 3′ overhang, which is necessary for t-loop formation and the structural integrity of the telomere. This process is carried out by the nuclease Apollo, which is bound to telomeric repeat-binding factor 2 (TRF2). Both the leading end and the lagging end of telomeres are further resected by exonuclease 1 (EXO1) to generate transient long overhangs. The CST (CTC1–STN1–TEN1) complex then binds to shelterin and mediates fill-in synthesis of the cytosine-rich strand (C-strand) at both ends. b | During development, telomerase is switched off through telomerase reverse transcriptase (TERT) silencing. As a result, telomeres experience the gradual attrition described in part a. After numerous population doublings, a few telomeres become too short (yellow) and lose their protective function. As a result, the kinases ATM and ATR are activated at the unprotected chromosome ends and this DNA damage response (DDR) signalling induces replicative arrest and senescence or apoptosis. This process limits the proliferative capacity of incipient cancer cells, thus functioning as a tumour suppressor pathway. Cells lacking p53 and RB function can avoid this replicative arrest.