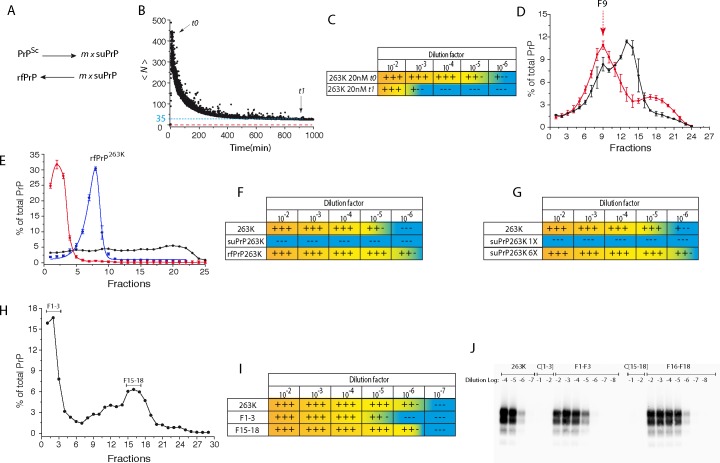
Fig 4. Equilibrium between PrPSc assemblies and suPrP.
(A) The occurrence of a quaternary structural transition in PrPSc assemblies suggests the existence of an equilibrium between PrPSc and suPrP. This equilibrium could be displaced by dilution. m represents the number of suPrP molecules that condense to form rfPrP. (B) The depolymerization of purified 263K PrPSc was explored using a quick dilution method from 1 μM to 10 nM. The variation in the size of PrPSc assemblies was monitored by static light scattering. As PrP assemblies are highly heterodisperses, weight-average molecular weights were estimated (see S5 Appendix for more details). PrP size is expressed in terms of the weight-averaged number of PrP protomers (<N>) and reported as a function of time. The fact that the relaxation of <N> does not reach <N> = 3 (estimated size of suPrP263K, dotted red line) suggests that the dilution factor (1 μM to 10 nM) is not sufficient to achieve total equilibrium displacement. (C) PMCA-templating activities of PrPSc assemblies at t0 and after size relaxation at the plateau (t1), as indicated with arrows in panel B and SI5 (n = 3). (D) Isopycnic concentration [36] of suPrP263K (S4 Appendix). The 263K-infected brain homogenate was treated with 6 M urea, then subjected to isopycnic sedimentation and analysed for PrP contents (red line). The density distribution of untreated 263K PrPSc is shown for comparison (black line). Fraction 1 corresponds to lowest density (top of the isopycnic gradient), and fraction 27 corresponds to the highest density (bottom of the gradient). (E) The F9 fraction of the isopycnic gradient, indicated with an arrow in (D), was subjected to SV (in blue) (n = 5 independent assays). For comparison, the sedimentation profiles of untreated 263K assemblies and suPrP before isopycnic concentration are represented with black and red lines, respectively. (F) The F9 fraction subjected to SV and corresponding to rfPrP263K was tested for templating activity in PMCA assays (n = 9). The templating activity of untreated 263K PrPSc and of suPrP (263K brain homogenate in 6M urea) was assessed in parallel runs. Refolding of suPrP into rfPrP was achieved using the urea dilution method. The 6-fold-concentrated suPrP fraction (suPrP263K 6X) in 6 M urea was directly diluted 10-fold, either in PMCA reaction medium to estimate its templating activity (G) or in SV loading buffer prior to SV for size distribution analysis (H). The resulting fractions 1–3 and 15–18 were analysed for templating activity via PMCA (I). As controls in the PMCA reaction, either the non-concentrated suPrP263K fraction in 6 M urea (suPrP263K 1X, G) or 263K PrPSc (I) was used. The panel (J) shows representative PMCA product analysed by Western Blot.