Abstract
Purpose
To evaluate the prevalence of large drusen in a uveitis clinic population.
Design
Retrospective, cohort study.
Methods
Patients with primary, non-infectious uveitis 55 years or older who were seen at the National Eye Institute of the National Institutes of Health from 2004 through August 2013 were reviewed using electronic medical records and photographic databases. Patients were classified as having age-related macular degeneration (AMD) if either eye had large drusen, geographic atrophy or neovascular AMD according to definitions used by the Eye Diseases Prevalence Research Group (EDPRG). The expected number of cases and standardised mortality ratio (SMR) for large drusen were estimated based on EDPRG estimates.
Results
We identified 177 patients aged ≥55 years as having primary non-infectious uveitis; 170 (96.0%) had gradable fundus photos. Average age was 65.0 ±7.5 years (range 55–87), and 87 were non-Hispanic white, 66 non-Hispanic black, 6 Hispanic white and 11 of other race/ethnicity. Large drusen were identified in four patients (2.4%; 95% CI 0.6 to 6.0). No patients were identified to have late AMD. In the uveitis cohort, the SMR for cases of large drusen, which was adjusted for age, was calculated to be 0.32 (95% CI 0.12 to 0.70) for the whole cohort, 0.28 (95% CI 0.09 to 0.79) for non-Hispanic whites and 0.46 (95% CI 0.14 to 1.29) for non-Hispanic blacks.
Conclusions
Large drusen prevalence among patients with uveitis ≥55 years of age appears less than the prevalence in the general US population after accounting for differences in age distribution, especially for non-Hispanic whites. Although the racial and gender distribution in this study population is not directly representative of the general US population, results of this study suggest possible sparing of patients with uveitis from AMD. A larger systematic study with greater power would be needed to confirm these findings.
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of irreversible vision loss among individuals greater than 65 years of age in the USA and other developed countries.1 It is estimated that more than seven million Americans have large drusen (≥125 µm) and are consequently at significant risk of developing late AMD. With the rapid growth of the US population over the age of 55, the burden of AMD will significantly increase in coming decades.
Many studies have indicated that inflammation may play a role in the pathogenesis of AMD. Inflammatory markers such as C reactive protein, activated microglia, activated complement fragments and aberrant complement activation have been associated with the disease.2–4 Notably, variants of complement factor H, a regulator of the alternative complement pathway, have been extensively studied and established to be associated with intermediate and late AMD.5–7 In addition to the innate immune system, the adaptive immune system has also been implicated in the development of AMD. Multiple studies have identified high titres of antiretinal antibodies in serum of individuals with early and late forms of AMD as compared with controls.8–10 Whether these autoantibodies are directly involved in the pathogenesis of AMD or a result of the disease process and consequential retinal damage is not clear. These observations implicate inflammation and the immune system in the complex multifactorial pathogenesis of AMD, though exact mechanisms have yet to be worked out. Because of the considerable interaction between the immune system and AMD, we aimed to investigate the prevalence of AMD among patients with uveitis and to explore potential explanations. To our knowledge, this is the first study exploring the prevalence of large drusen or AMD among patients with uveitis.
PATIENTS AND METHODS
This study is a retrospective, cohort study. A review of patients aged 55 years and older who had primary, noninfectious uveitis and were seen in the uveitis clinic at the National Eye Institute (NEI) of the National Institutes of Health from January 2004 through August 2013 was performed. The Office of Human Subjects Research at the National Institutes of Health approved this study.
Information was obtained from electronic medical records and photographic databases. Fundus photographs were taken and available for almost all patients seen at the uveitis clinic of the NEI. Images were taken from the first and last visit, if available, and the best image was used for grading if there were multiple visits within a year’s time. For the purposes of this study, we excluded patients with posttraumatic and postsurgical uveitis and other secondary uveitides including infectious uveitis, intraocular malignancy and autoimmune retinopathy. In addition, patients with colour fundus photographs in which the fundus could not be adequately visualised due to media opacities or poor dilation were excluded. All patients had a record of a full ophthalmic examination, dilated colour fundus photography, records of systemic work-up for systemic associations of uveitis where indicated and records of past and current immunosuppressive treatment for their disease. AMD was identified by examination of dilated, non-stereoscopic, colour fundus photographs. Additional imaging modalities such as fluorescein angiography (FA), fundus autofluorescence (FAF) and optical coherence tomography (OCT) were sometimes available and used to aid in grading, for example, to rule out choroidal neovascularisation. Almost all patients (165/170) had FA and/or FAF at the same time of the fundus photography (FAF images unavailable prior to 2007). OCT was used on request and available for eight of the nine cases which were adjudicated after initial grading (OCT images unavailable prior to 2007).
The definitions for large drusen, geographic atrophy (GA) and neovascular AMD (NV AMD) used in this study were the same definitions used in the Eye Diseases Prevalence Research Group (EDPRG) study on the prevalence of AMD in the USA to allow for a reasonable comparison of prevalence.1 Large drusen were defined as drusen 125 µm or larger in diameter in the macula, which was defined as a region 3000 µm in diameter centred on the foveola, in either or both eyes. As in the EDPRG study, definitions for GA and NV AMD were defined according to the International ARM Study Group definitions.11 GA was defined as a discrete area of retinal depigmentation at least 175 µm in diameter with a sharp border and visible choroidal vessels in the absence of NV AMD in the same eye. NV AMD was defined as serous or haemorrhagic detachment of either the retinal pigment epithelium or sensory retina, the presence of subretinal fibrous tissue or minimal subretinal fibrosis and widespread retinal pigment epithelial atrophy. In addition, medium-sized drusen was defined as drusen size 63 to less than 125 µm and was included in this study as it has been recognised to increase one’s risk of progression to large drusen, pigmentary changes, and consequently late AMD.12,13
Gradable fundus photos were examined twice by separate examiners (HNS, CM), and the presence of large drusen and medium drusen as well as GA or NV AMD was recorded. Subsequently, the initial examiners reviewed cases with FA, FAF and OCT when necessary and available. Graders were initially masked to uveitic diagnoses. However, if requested, uveitic diagnoses were provided to graders to consider in their assessment. For example, in an image from a patient with multifocal choroiditis, the grader may suspect scarring to be due to underlying uveitis and request the uveitic diagnosis. In the absence of drusen and presence of such atrophic scarring related to multifocal choroiditis, the image would be graded as no AMD. Disagreements in grading were adjudicated by two additional examiners (EYC, RBN) using colour fundus photos, FA, FAF and OCT (when available).
Electronic and paper medical records were used to determine types and duration of immunomodulatory use and the anatomic location of uveitis. Because of the retrospective nature of this study and the difficulty in determining exact treatment duration, we used a categorical classification for treatment duration, separating patients based on immunomodulatory therapy use for less than or equal to 1 year and greater than 1 year. Types of immunomodulatory therapy included systemic corticosteroids, non-biological immunosuppressives (such as antimetabolites, T-cell inhibitors and cytotoxic agents) or biological immunosuppressives. Local therapy included corticosteroid eye drops used greater than 3 months, any corticosteroid injections or intravitreal corticosteroid implants. Anatomic location of uveitis was classified according to the Standardization of Uveitis Nomenclature Working Group.14
Statistical analyses
Because of the small numbers within the age strata and the differences in age distribution, we computed age-adjusted rates by using indirect age adjustment, with the summary rate described as a standardised mortality ratio (SMR). An SMR of 1 indicates that the observed and expected numbers of events are the same. An SMR <1 indicates that the observed number of events is fewer than expected, while SMR >1 indicates that the observed number of events is more than expected. For the indirect age standardisation, we applied the US age-specific rates for large drusen1 to our uveitis population in each age stratum to estimate the expected number of large drusen cases. This age adjustment was performed for the overall group and also separately for the non-Hispanic white and black subgroups. In addition, we applied the US age-specific rates for late AMD to our uveitis population to estimate the expected number of late AMD cases given our cohort size and age distribution.
We stratified and reported prevalence rates of large drusen within our cohort by 5 year age strata as the age distribution in our cohort differed from that of the US population. The proportion of large drusen cases in the US population for individuals 55 years or older was estimated by using the numerators reported by Friedman et al1 with denominators reported in the 2000 US Census.15 However, the numbers in our uveitis cohort are too small to make a meaningful comparison to the US population for each age strata. For each of the age-specific prevalence rates in our uveitis group, we computed 95% CIs based on the Poisson distribution, and we computed 95% CIs for the age-specific US prevalence figures using the modified Wald method.
To explore differences in the prevalence of large drusen among subjects with different anatomic types of uveitis, we compared subjects with anterior segment uveitis and posterior segment uveitis, including intermediate, posterior and panuveitis. To explore possible differences in the prevalence of large drusen based on treatment duration, we compared subjects with less than or equal to 1 year and greater than 1 year of treatment. We compared the percentage with large drusen between subgroups based on the anatomic type of uveitis and treatment duration using two-tailed Fisher’s exact test.
RESULTS
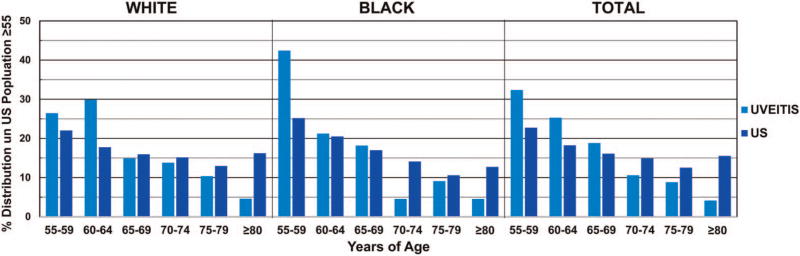
Two hundred and twenty-nine patients ≥55 years of age, seen by the uveitis service at NEI from January 2004 through August 2013, were reviewed. Of these patients, 177 were identified with primary non-infectious uveitis. Digital fundus images for 170 patients were gradable (96.0%). Average age was 65.0±7.5 years (range 55.1–87.2). Eighty seven of the 170 patients were non-Hispanic white (51.2%), 66 were non-Hispanic black (38.8%), 6 were Hispanic white and 11 were of other race/ethnicity. One hundred and thirteen were female (66.5%), and 57 were male (table 1). Comparing the age and race distribution in our uveitis cohort with that of the general US population, patients of the younger age subgroups were relatively overrepresented, by as much as 17.2% in the non-Hispanic black 55–59 age group, and patients of the older age subgroups were relatively underrepresented, by as much as 11.6% in the non-Hispanic white ≥80 age group (figure 1).
Table 1.
Baseline characteristics of patients with uveitis
| Characteristic | Result |
|---|---|
| Patients with uveitis (n) | 170 |
| Age (years) | |
| Mean (SD) | 65.0 (7.5) |
| Range | 55.1–87.2 |
| Sex (n, %) | |
| Male | 57 (33.5) |
| Female | 113 (66.5) |
| Race/ethnicity (n, %) | |
| Non-Hispanic white | 87 (51.2) |
| Non-Hispanic black | 66 (38.8) |
| Hispanic white | 6 (3.5) |
| Other | 11 (6.5) |
| Uveitis anatomic subtype* (n, %) | |
| Anterior | 48 (28.2) |
| Intermediate | 40 (23.5) |
| Posterior or panuveitis | 82 (48.2) |
| Duration of uveitis† (years) | |
| Mean (SD) | 9.8 (10.5) |
| Range | 0.0–49.3 |
| Systemic immunomodulatory therapy duration‡ (n, %) | |
| >1 year | 77 (45.3) |
| ≤1 year | 93 (54.7) |
Anatomic subtype of uveitis was classified according to Standardization of Uveitis Nomenclature Working Group definitions.14
Duration of uveitis could not be determined for two patients, one with anterior uveitis and one with panuveitis.
Systemic immunomodulatory therapy included systemic corticosteroids, non-biological immunosuppressives (such as antimetabolites, T-cell inhibitors and cytotoxic agents) or biological immunosuppressives.
n, number.
Figure 1.
Comparison of age distribution: uveitis cohort versus US population ≥55 years. The age distribution between the uveitis cohort and US population was compared and shows that patients of the younger age subgroups were relatively overrepresented in the uveitis cohort, while patients of the older age subgroups were relatively underrepresented. Numbers based on age and race were calculated from 2000 US census data.
Percentages represent the percentage that each age subgroup is represented among all individuals ≥55 in each respective population to allow for comparison.
Of the 170 patients with uveitis, 4 (2.4%; 95% CI 0.6 to 6.0) were identified to have large drusen, and none had GA or NV AMD. The ages of the patients with uveitis and large drusen were 61, 63, 82 and 87. In all four patients, large drusen were present in only one eye, and in two of these patients, only one single large druse was present. Medium-sized drusen were identified in 15 patients (8.8%).
While adjusting for age, we computed the number of large drusen cases that would be expected in the uveitis population if the US rates were applied (table 2). For the entire uveitis cohort, the number of expected cases was 12.49. The SMR (observed/expected) was 0.32 (95% CI 0.12 to 0.70), indicating an observed large drusen rate of about one-third that of the expected rate and a CI indicating an observed large drusen rate from 30% to 88% less than the expected rate. The number of expected cases of large drusen in non-Hispanic whites was 7.03, resulting in an SMR of 0.28 (95% CI 0.09 to 0.79). In non-Hispanic blacks, the number of expected cases of large drusen was 4.31, resulting in an SMR of 0.46 (95% CI 0.14 to 1.29). By using the same method, we determined that the number of expected cases of late AMD in our cohort, given our cohort size and age distribution, was 1.53. With this low expected rate and no cases of late AMD in our population, we are unable to make any meaningful conclusions on the relative risk of late AMD in our uveitis population. We stratified the overall cohort by 5 year age groups and race; however, our numbers are too small to make a meaningful comparison to the US population (see online supplementary table S1).
Table 2.
Number of observed and expected cases of large drusen adjusting for age distribution and by race
| Number of large drusen cases | |||
|---|---|---|---|
|
|
|||
| Race | Observed | Expected* | SMR (95% CI)† |
| White | 2 | 7.03 | 0.28 (0.09 to 0.79) |
| Black | 2 | 4.31 | 0.46 (0.14 to 1.29) |
| All | 4 | 12.49 | 0.32 (0.12 to 0.70) |
Expected number of large drusen cases were estimated using indirect age adjustment, in which we applied the US age-specific rates for large drusen1 to our uveitis population in each age stratum.
SMR (standardised mortality ratio): An SMR of 1 indicates that the observed number of events is equal to the expected number of events if the US population rates held in the uveitis population. An SMR <1 indicates that the observed number of events is fewer than expected, while SMR >1 indicates that the observed number of events is more than expected. 95% CIs for SMR values are calculated based on the Poisson distribution.
Of the 170 patients, 48 had anterior uveitis, 40 had intermediate uveitis and 82 had posterior or panuveitis. Average time from initial uveitis diagnosis to fundus imaging was 9.8 ±10.5 years (range 0.0–49.3). The date of initial uveitis diagnosis, and therefore duration of uveitis, could not be determined for two patients (one patient with anterior uveitis and another with panuveitis). The proportion with large drusen, stratifying by uveitis subtype, was 3/48 for anterior uveitis and 1/82 for posterior or panuveitis; large drusen were not identified in any of the 40 patients with intermediate uveitis. Average duration of uveitis was 8.4±9.5 years (range 0.0–34.0) for patients with anterior uveitis, and 10.4±10.8 years (range 0.0–49.3) for patients with posterior segment uveitis. A statistical analysis comparing large drusen prevalence between patients with anterior uveitis (3/48, 6.2%) and posterior segment uveitis (1/122, 0.8%) was not statistically significant (p=0.07). Patients subgrouped by anterior uveitis and posterior segment uveitis were stratified by age and race (see online supplementary table S2).
Regarding treatment duration in our uveitis cohort, 77 (45.3%) received systemic immunomodulatory therapy >1 year, and 138 (81.2%) received local steroid therapy. A statistical analysis comparing large drusen prevalence between longer (>1 year: 3/77, 3.9%) and shorter (≤1 year: 1/93, 1.1%) duration of systemic immunomodulatory use showed no statistically significant difference (p=0.33). Patients subgrouped based on receiving systemic immunomodulatory therapy >1 or ≤1 year were stratified by age and race (see online supplementary table S3).
Of the four patients in whom large drusen were identified, three received systemic immunomodulatory therapy for >1 year and one for 3 months. In these patients with large drusen, types of systemic immunomodulatory therapies used varied widely, including prednisone, methylprednisolone, methotrexate, mycophenolate mofetil, ciclosporin, azathioprine, efalizumab, etanercept, adalimumab and infliximab. All four patients with large drusen received local therapy in the form of prednisolone acetate drops.
DISCUSSION
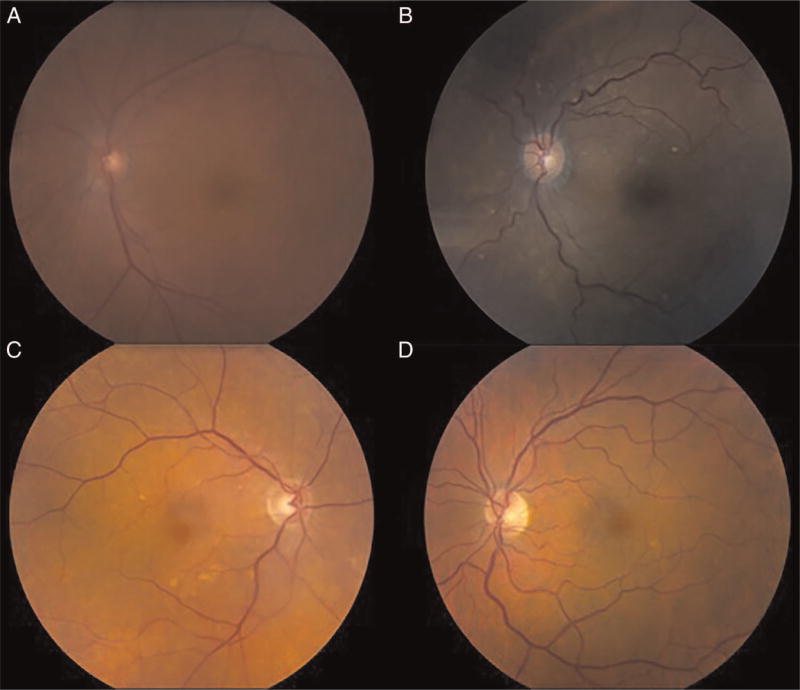
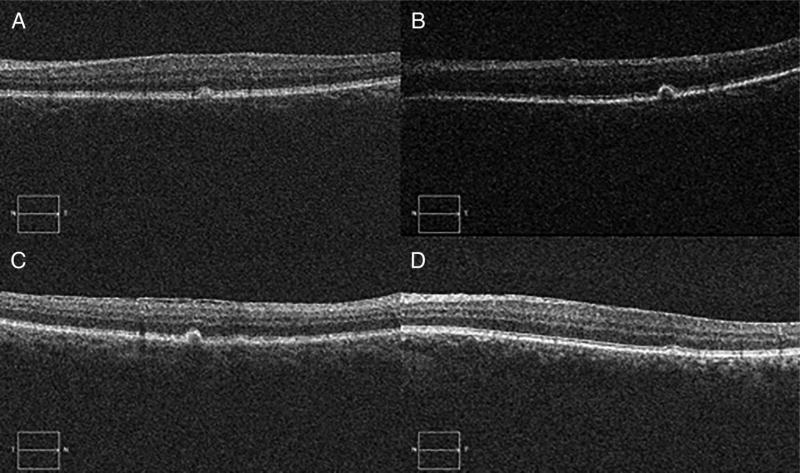
This study suggests that the prevalence of large drusen may be lower among patients with uveitis than in the general US population. When adjusted for age, the number of observed cases of large drusen in our cohort was significantly less than would be expected given the age distribution of the entire uveitis cohort (SMR=0.32; 95% CI 0.12 to 0.70) and of non-Hispanic whites (SMR=0.28; 95% CI 0.09 to 0.79). While the number of observed cases of large drusen among non-Hispanic blacks in our cohort was less than would be expected given the age distribution of our cohort, this difference was not statistically significant (SMR=0.46; 95% CI 0.14 to 1.29). Of the four patients in whom large drusen were identified, drusen were seen in only one eye. In two patients, only a single druse with sharply defined, discrete borders could be seen by colour fundus images (figure 2A–D). This can also be seen on OCT, which was available for all four patients in whom large drusen were identified (figure 3A–D). This study did not have power to investigate late AMD, because no cases were identified, and only 1.53 cases would have been expected given our cohort size and age distribution. The relative overrepresentation of non-Hispanic black persons in our cohort may have contributed to the absence of late AMD, which is less frequent in the black population.1
Figure 2.
Colour fundus photographs of four cases of large drusen in patients with uveitis. (A–D) Fundus photos of patients in whom large drusen were identified: (A) A 63-year-old non-Hispanic black female with anterior uveitis of 7 months duration, (B) A 82-year-old non-Hispanic black female with anterior uveitis of 3 years duration, (C) A 87-year-old non-Hispanic white female with panuveitis of 27 years duration and (D) A 61-year-old non-Hispanic white male with anterior uveitis of 34 years duration.
Figure 3.
Optical coherence tomography (OCT) of four cases of large drusen in patients with uveitis. (A–D) OCT of patients in whom large drusen were identified: (A) A 63-year-old non-Hispanic black female with anterior uveitis of 7 months duration, (B) A 82-year-old non-Hispanic black female with anterior uveitis of 3 years duration, (C) A 87-year-old non-Hispanic white female with panuveitis of 27 years duration and (D) A 61-year-old non-Hispanic white male with anterior uveitis of 34 years duration.
There is a substantial body of evidence suggesting that various inflammatory pathways play a role in the pathogenesis of AMD as mentioned earlier. Inflammatory markers in both peripheral blood and ocular lesions of AMD have been identified.2–10 The suggested sparing from AMD in this uveitis cohort may be due to multiple factors, including long-term immunomodulatory treatment or factors unique to the pathogenesis of uveitis and AMD. In our cohort, 65.3% had received systemic immunomodulatory therapy and 81.2% had received local steroid therapy. A comparison of the large drusen prevalence based on duration of systemic immunomodulatory use (>1 vs ≤1 year) was not statistically significant (p=0.33); however, our numbers are small to make conclusions and the use of categorisation for duration of treatment may limit our interpretation. Though not definitive or fully consistent, some studies have suggested a potential benefit of immunomodulatory drug use in AMD. In a study evaluating the prevalence of AMD among patients with rheumatoid arthritis, another immune-mediated disease, McGeer and Sibley16 found that individuals with rheumatoid arthritis appeared to be relatively spared from AMD, identifying three cases of AMD in 993 patients with rheumatoid arthritis. Though ascertainment of AMD was through clinical examination by an ophthalmologist and imaging was not used as in other prevalence studies or our study (personal communication, Dr Patrick McGeer), this remains a provocative finding. It was suggested that this sparing may be due to long-term anti-inflammatory treatment although genetic and environmental factors could not be ruled out. The association between anti-inflammatory drug use and AMD has been explored in several epidemiological studies and randomised trials; however, findings have been inconsistent.17–22 More recently, in a pilot trial our group reported a favourable effect of systemic immunosuppressive treatment in patients with NV AMD.23 Although this trial was not definitively powered, this study provides supporting evidence that suppressing inflammation systemically may be beneficial in the management of AMD. Further studies are needed to explore the effect of immunomodulatory use on the pathogenesis and clinical course of AMD.
The difference in large drusen prevalence between posterior segment uveitis (1/122, 0.8%) and anterior uveitis (3/48, 6.2%) was not statistically significant (p=0.07). Though our small numbers limit a meaningful interpretation, we find them compelling along with observations of related studies. Some have suggested that some inflammatory cells, that is, macrophages, may be favourable in the pathogenesis of AMD.24,25 Further, randomised controlled trials have shown regression of drusen after eyes were treated with laser photocoagulation, and it was suggested that drusen regression may result from the localised inflammatory response and subsequent phagocytosis.26,27 Though these observations are provocative in light of our study, we are unaware of a clear mechanism which may explain any potential differences in large drusen prevalence among patients with anterior uveitis and posterior segment uveitis.
Several limitations are present in this retrospective study. Our study includes a small number of patients and is not sufficiently powered to be conclusive. Patients younger than 55 years of age were excluded because of the low expected rate of large drusen in this age group. Additionally, due to the tertiary care nature of our institution, we often receive patients with active, severe, intraocular inflammation, which makes the macula difficult to visualise. Patients in whom the macula could not be visualised due to media opacities or poor dilation were excluded from our cohort. For these patients with fundus photos which graders deemed ungradable, we cannot know whether these patients had large drusen or late AMD, which may have affected the prevalence of AMD in our cohort. Another limitation due to the tertiary care nature of our institution, patients with anterior uveitis were underrepresented in our cohort as compared with the US population. As there appeared to be a higher prevalence of large drusen in patients with anterior uveitis in our study, we cannot know whether the underrepresentation of anterior uveitis may have affected the overall prevalence of large drusen in our study. A larger study with a greater proportion of patients with anterior uveitis would be informative.
As the disease processes of many uveitidies may disrupt the macula, graders were given the uveitis diagnoses on request to aid in their assessment. In the absence of drusen and in the presence of pathology characteristic of a given uveitis diagnosis, the image would be graded as no AMD. However, we cannot rule out ascertainment bias in such cases. Another limitation arises from the age and race distribution in our cohort, which is not representative of the US population. In calculating the number of expected cases of large drusen in our cohort, we adjusted for the younger age distribution of our cohort by using indirect age standardisation. Regarding race, non-Hispanic whites are relatively underrepresented in our cohort and may have affected the findings of this study. This underrepresentation of non-Hispanic whites may be partly due to the race predilection of the uveitides present in our cohort, such as sarcoidosis which is more common among blacks.28 Lastly, in addition to fundus photos, we also used FAF, FA, and OCT to detect large drusen, which were not used in the studies to which we are comparing prevalence. Though we cannot be certain, the use of multiple imaging modalities may have led to an underestimation or overestimation of large drusen when compared with the methods used by other studies to estimate the prevalence of large drusen.
In conclusion, this study suggests that the prevalence of large drusen among patients with uveitis may be lower than the prevalence in the general US population, especially for non-Hispanic whites. However, as our cohort is limited by size and not directly representative of the US population, a larger systematic study is needed to confirm the findings of this study.
Supplementary Material
Acknowledgments
Funding This work is supported by the NEI Intramural Research Program and the NIH Medical Research Scholars Program. The NIH Medical Research Scholars Program is a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer, the Doris Duke Charitable Foundation, the Newport Foundation, the American Association for Dental Research, the Howard Hughes Medical Institute and the Colgate-Palmolive Company, as well as other private donors. For a complete list, please visit the Foundation website at http://fnih.org/work/education-training-0/medical-research-scholars-program.
Footnotes
Contributors ARF contributed to the conception or design of the study, data collection, analysis and interpretation of findings, statistical expertise, obtaining funding, literature search, administrative support, writing the manuscript and final approval of the manuscript. EYC contributed to the conception or design of the study, analysis and interpretation of findings, statistical expertise, literature search, critical revision of the manuscript and final approval of the manuscript. CM contributed to the conception or design of the study, data collection, analysis and interpretation of findings, literature search, critical revision of the manuscript and final approval of the manuscript. SV contributed to the conception or design of the study, data collection, analysis and interpretation of findings, statistical expertise, literature search, administrative support, writing and critical revision of the manuscript and final approval of the manuscript. FLF contributed to the conception or design of the study, analysis and interpretation of findings, statistical expertise, obtaining funding, literature search, critical revision of the manuscript and final approval of the manuscript. RBN contributed to the conception or design of the study, analysis and interpretation of findings, statistical expertise, literature search, obtaining funding, administrative support, provision of patients and resources, critical revision of the manuscript and final approval of the manuscript. HNS contributed to the conception or design of the study, data collection, analysis and interpretation of findings, statistical expertise, literature search, obtaining funding, administrative support, provision of patients and resources, writing and critical revision of the manuscript and final approval of the manuscript.
Competing interests None declared.
Ethics approval The Office of Human Subjects Research, National Institutes of Health.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Both the first and senior authors had full access to all the data in the study. Additional available data are included in online supplementary material.
References
- 1.Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DH, Mullins RF, Hageman GS, et al. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 3.Ma W, Zhao L, Wong WT. Microglia in the outer retina and their relevance to pathogenesis of age-related macular degeneration. Adv Exp Med Biol. 2012;723:37–42. doi: 10.1007/978-1-4614-0631-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson LV, Leitner WP, Staples MK, et al. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–96. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 5.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 7.Edwards AO, Ritter R, III, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 8.Morohoshi K, Ohbayashi M, Patel N, et al. Identification of anti-retinal antibodies in patients with age-related macular degeneration. Exp Mol Pathol. 2012;93:193–9. doi: 10.1016/j.yexmp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Patel N, Ohbayashi M, Nugent AK, et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115:422–30. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joachim SC, Bruns K, Lackner KJ, et al. Analysis of IgG antibody patterns against retinal antigens and antibodies to alpha-crystallin, GFAP, and alpha-enolase in sera of patients with “wet” age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245:619–26. doi: 10.1007/s00417-006-0429-9. [DOI] [PubMed] [Google Scholar]
- 11.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–74. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 12.Chew EY, Clemons TE, Agrón E, et al. Age-Related Eye Disease Study Research Group. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol. 2014;132:272–7. doi: 10.1001/jamaophthalmol.2013.6636. [DOI] [PubMed] [Google Scholar]
- 13.Ferris FL, III, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Census Bureau. Census 2000 PHC-T-9. Population by Age, Sex, Race, and Hispanic or Latino Origin for the United States, 2000. [accessed 23 Jun 2015]; http://www.census.gov/population/www/cen2000/briefs/phct9/tables/tab01.pdf.
- 16.McGeer PL, Sibley J. Sparing of age-related macular degeneration in rheumatoid arthritis. Neurobiol Aging. 2005;26:1199–203. doi: 10.1016/j.neurobiolaging.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Clemons TE, Milton RC, Klein R, et al. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533–9. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong PT, Chakravarthy U, Rahu M, et al. Associations between aspirin use and aging macula disorder: the European Eye Study. Ophthalmology. 2012;119:112–18. doi: 10.1016/j.ophtha.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Klein BE, Howard KP, Gangnon RE. Long-term use of aspirin and age-related macular degeneration. JAMA. 2012;308:2469–78. doi: 10.1001/jama.2012.65406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liew G, Mitchell P, Wong TY, et al. The association of aspirin use with age-related macular degeneration. JAMA Intern Med. 2013;173:258–64. doi: 10.1001/jamainternmed.2013.1583. [DOI] [PubMed] [Google Scholar]
- 21.Christen WG, Glynn RJ, Ajani UA, et al. Age-related maculopathy in a randomized trial of low-dose aspirin among US physicians. Arch Ophthalmol. 2001;119:1143–9. doi: 10.1001/archopht.119.8.1143. [DOI] [PubMed] [Google Scholar]
- 22.Christen WG, Glynn RJ, Chew EY, et al. Low-dose aspirin and medical record-confirmed age-related macular degeneration in a randomized trial of women. Ophthalmology. 2009;116:2386–92. doi: 10.1016/j.ophtha.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nussenblatt RB, Byrnes G, Sen HN. A randomized pilot study of systemic immunosuppression in the treatment of age-related macular degeneration with choroidal neovascularization. Retina. 2010;30:1579–87. doi: 10.1097/IAE.0b013e3181e7978e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao X, Shen D, Patel MM, et al. Macrophage polarization in the maculae of age-related macular degeneration: A pilot study. Pathol Int. 2011;61:528–35. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combadière C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parodi MB, Virgili G, Evans JR. Laser treatment of drusen to prevent progression to advanced age-related macular degeneration. Cochrane Database Syst Rev. 2009;(3):CD006537. doi: 10.1002/14651858.CD006537.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Duvall J, Tso MM. Cellular mechanisms of resolution of drusen after laser coagulation: an experimental study. Arch Ophthalmol. 1985;103:694–703. doi: 10.1001/archopht.1985.01050050086024. [DOI] [PubMed] [Google Scholar]
- 28.Nussenblatt RB, Whitcup SM. Uveitis: fundamentals and clinical practice. 4. Philadelphia: Mosby; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.