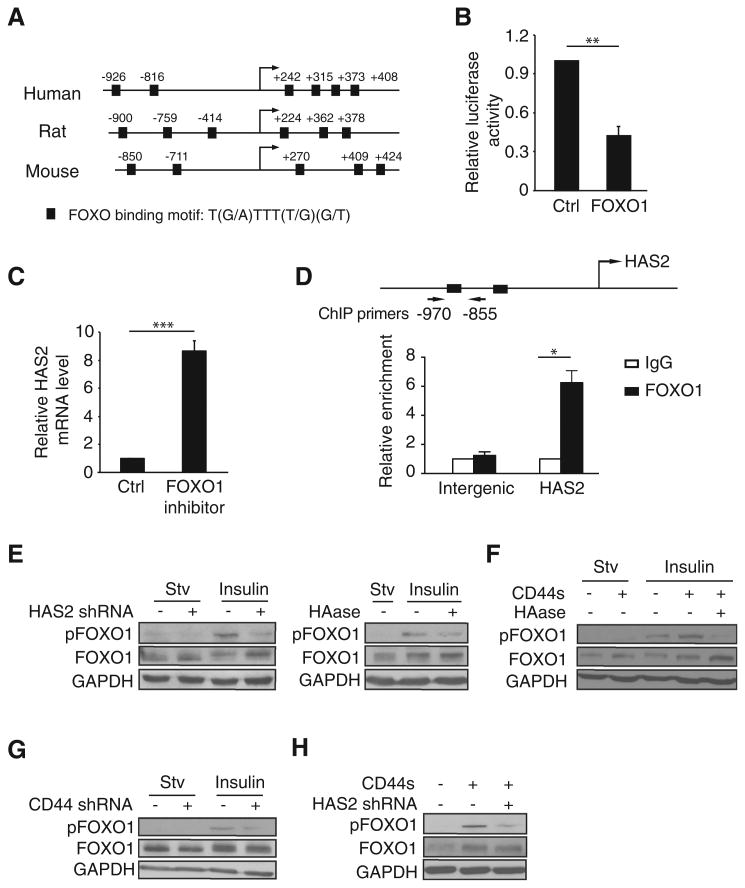
Figure 6.
HAS2 transcription is repressed by FOXO1, whose activity is inhibited by CD44s and HAS2. A, Schematic of the HAS2 promoter showing conserved FOXO1 putative binding sites in human, rat, and mouse. Arrow, transcription start site. B, Relative luciferase activity in 293FT cells cotransfected with FOXO1 and the HAS2 promoter luciferase reporter constructs. C, qRT-PCR analysis of endogenous HAS2 levels in control and FOXO1 inhibitor-treated cells (AS1842856, 0.5 μmol/L). D, Quantitative ChIP analysis of the relative occupancy of FOXO1 at the HAS2 promoter in 293FT cells overexpressing FOXO1. As a negative control, enrichment at an amplicon located in an intergenic region was assayed. Bar graphs show averages of three independent ChIP experiments. E, Immunoblot analysis of pFOXO1 in HAS2-depleted (left) or HAase-treated (100 μg/mL) LM2 cells, followed by insulin stimulation (10 μg/mL; 30 minutes). F, Immunoblot analysis of pFOXO1 in Mes10A cells, CD44s-overexpressing Mes10A cells, and HAase-treated (35 μg/mL) CD44s-overexpressing Mes10A cells, followed by insulin stimulation (10 μg/mL; 30 minutes). G, Immunoblot analysis of pFOXO1 levels in control and CD44s-depleted Mes10A cells upon insulin stimulation (10 μg/mL; 30 minutes). H, Immunoblot analysis of pFOXO1 levels in control, CD44s-overexpressing, and CD44s-overexpressing and HAS2-depleted Mes10A cells, followed by insulin stimulation (10 μg/mL; 30 minutes). Error bars (B, C, and D), SD; n = 3. *, P < 0.05; ***, P < 0.001.