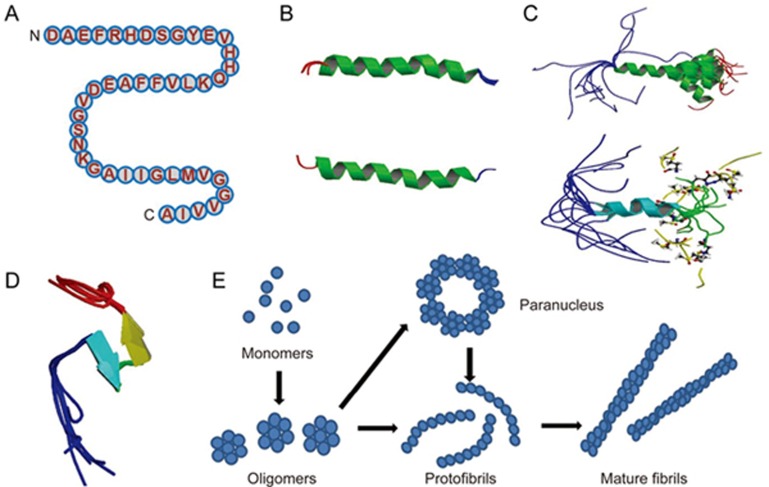
Figure 3.
Structures of Aβ monomer, fibril and oligomers. (A) The primary amino acid sequence of the 42 amino acid Aβ isoform Aβ42. Aβ encompasses a group of peptides ranging in size from 37–49 residues. (B) The structure of amyloid beta peptide (1–28), which forms a predominately alpha-helical structure that can be converted to a beta-sheet structure in membrane-like media (PDB code: 1AMC, 1AMB), it's the major proteinaceous component of amyloid deposits in Alzheimer's disease. The side chains of histidine-13 and lysine-16 residing on the same face of the helix are close. (C) Solution structure of amyloid beta peptide (1–40), in which the C-terminal two-thirds of the peptide form an alpha-helix conformation between residues 15 and 36 with a kink or hinge at 25–27 in aqueous sodium dodecyl sulfate (SDS) micelles with a bend centered at residue 12, while the peptide is unstructured between residues 1 and 14 which are mainly polar and likely solvated by water (PDB code: 1BA4, 1BA6) . It collapsed into a compact series of loops, strands, and turns with no alpha-helical or beta-sheet structure. The van der Waals and electrostatic forces maintain its conformational stabilization. Approximately 25% of the surface is uninterrupted hydrophobic, and the compact coil structure is meta-stabled, which may lead to a global conformational rearrangement and formation of intermolecular beta-sheet secondary structure caused by fibrillization. (D) Amyloid beta peptide (10–35) forms a collapsed coil structure (PDB code: 1HZ3). It collapsed into a compact series of loops, strands, and turns with no alpha-helical or beta-sheet structure. The van der Waals and electrostatic forces maintain its conformational stabilization. Approximately 25% of the surface is uninterrupted hydrophobic, and the compact coil structure is meta-stabled, which may lead to a global conformational rearrangement and formation of intermolecular beta-sheet secondary structure caused by fibrillization. (E) Proposed pathway for the conversion of amyloid beta monomers to higher order oligomers, protofibrils and fibrils. Aβ monomers can form higher order assemblies ranging from low molecular weight oligomers, including dimers, trimers, tetramers, and pentamers, to mid-range molecular weight oligomers including hexamers, nonamers and dodecamers to protofibrils and fibrils.