Table 1. Optimization of reaction conditions a .
| ||||||
| Entry | Ligand | Solvent | Conv b (%) | 1a | 2a | S e |
| ee c (%) | ee c , d (%) | |||||
| 1 | L1 | THF | 64 | 98 | 60 | 17 |
| 2 | L1 | Toluene | 71 | 99 | 42 | 11 |
| 3 | L1 | DME | 43 | 63 | 74 | 12 |
| 4 | L1 | DCE | 44 | 45 | 57 | 5 |
| 5 | L2 | THF | 53 | 92 | 83 | 29 |
| 6 | L3 | THF | 20 | 7 | 50 | 1 |
| 7 | L4 | THF | 21 | 24 | 88 | 25 |
| 8 | L5 | THF | 29 | 38 | 95 | 40 |
| 9 | L6 | THF | 51 | 99.4 | 97 | 251 |
| 10 | L7 | THF | 37 | 37 | 64 | 6 |
| 11 | L8 | THF | 39 | 23 | 36 | 3 |
| 12 | L9 | THF | 31 | 5 | 31 | 2 |
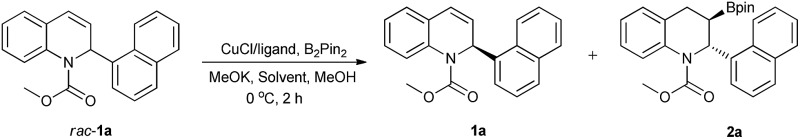
aReaction conditions: CuCl (0.025 mmol), ligand (0.025 mmol), rac-1a (0.5 mmol), B2Pin2 (0.6 mmol), MeOK (0.1 mmol), solvent (1.5 mL) MeOH (1.0 mmol), 0 °C, 2 h.
bCalculated conversion, C = ee1a/(ee1a + ee2a).
cDetermined by chiral HPLC and SFC analysis.
dDiastereomeric ratio (dr) > 99 : 1 (determined by 1H NMR).
eSelectivity factor (s) = ln[(1 – C) (1 – ee1a)]/ln[(1 – C) (1 + ee1a)].
