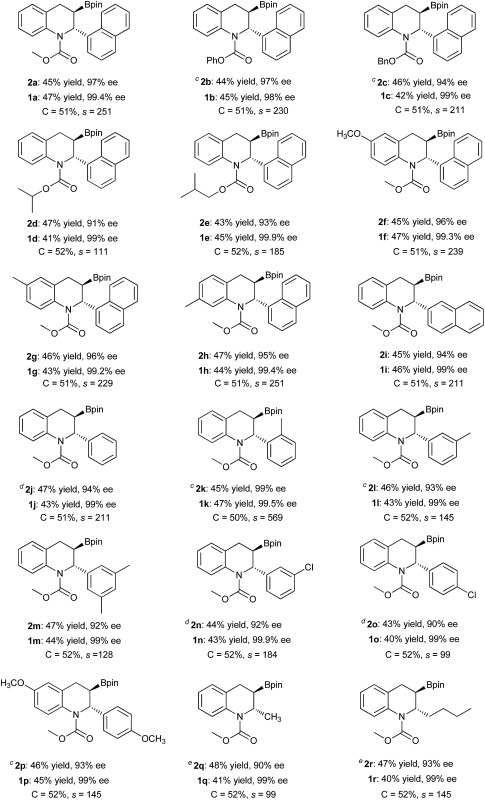
Table 2. Substrate scope a , b .
|
|
aUnless otherwise mentioned, all reactions were performed with CuCl (0.025 mmol), (R,S p)-JosiPhos-1 (0.025 mmol), rac-1 (0.5 mmol), B2Pin2 (0.6 mmol), MeOK (0.1 mmol), THF (1.5 mL), MeOH (1.0 mmol), 0 °C, 30 min.
bIsolated yield; calculated conversion, C = ee1/(ee1 + ee2); enantiomeric excess (ee) was determined by chiral HPLC or SFC analysis using a chiral stationary phase; diastereomeric ratio (dr) > 99 : 1 (determined by 1H NMR). Selectivity factor (s) = ln[(1 – C) (1 – ee1)]/ln[(1 – C) (1 + ee1)].
cTHF/toluene/DME = 2 : 1 : 1 (1.5 mL), 2 h.
d tBuOK (0.1 mmol), THF/toluene/DME = 1 : 1 : 1 (1.5 mL), 2 h.
eTHF/toluene/DME = 1 : 1 : 1 (1.5 mL), 2 h.
