Table 1.
Solution metathesis reactions of Ph2IOTf.
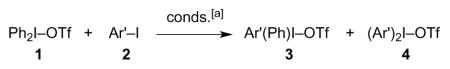
| ||||
|---|---|---|---|---|
| entry | Ar’ | time (h) | ratio 3:4:1[b] | yield[c] |
| a | 4-Me-C6H4 | 24 | 9.8:28.5:1.0 | 48 |
| b | 4-MeO-C6H4 | 25 | 27.3:23.1:1.0 | 78 |
| c | 1-naphthyl | 26 | 2.0:1.0:0.0 | 14 |
Conditions: 0.2 M solution of Ph2IOTf in (CH2Cl)2, 5 equiv aryl iodide, thick-walled glass tube sealed with a Teflon screwcap and immersed in an oil bath kept at 120–125 °C.
Molar ratios calculated by integration of 1H NMR spectra.
Percent yield after silica gel column chromatography (gradient 10% → 40% acetone-CH2Cl2) to remove nonpolar byproducts. The stated value is the sum of the yields of individual compounds present in the product mixture. A comparison of 1H NMR spectra of crude and purified reaction mixtures indicated that insignificant changes in the ratio of iodonium triflate products had occurred upon chromatography.
