Table 3.
Metathesis reactions of iodonium triflates 7.
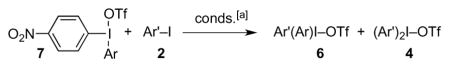
| ||||
|---|---|---|---|---|
| entry | Ar | Ar’ | ratio 6:4:7:(other)[b] | yield[c] |
| a[d] | Ph | Ph | 12.9[e]:1.0:(−)[f] | 65 |
| b[d] | “ | 4-MeC6H4 | 1.0:1.3:0.0:(−)[f] | 73 |
| c[d] | “ | 4-MeOC6H4 | 60.1:35.1:1.0:(−)[f] | 76 |
| d[d] | “ | 1-naphthyl | 33.3:5.9:1.0:(−)[f] | 59 |
| e[d] | 4-MeOC6H4 | Ph | 11.5:8.2:1.0:(1.0)[g] | 60 |
| f[d] | “ | 4-MeC6H4 | 1.3:1.0:0.0:(−)[f] | 79 |
| g[d] | “ | 4-MeOC6H4 | 6.9[e]:1.0:(−)[f] | 89 |
| h[d] | “ | 1-naphthyl | 8.4:1.2:1.2:(1.0+0.6)[h] | 64 |
| i[d] | mesityl | 4-MeC6H4 | 1.0:7.0:0.0:(−)[f] | 26 |
| j[d] | “ | 4-MeOC6H4 | 1.0:13.0:0.0:(−)[f] | 37 |
| k[d] | “ | 1-naphthyl | 1.0:8.4:8.4:(−)[i] | 27 |
: same as in Table 1.
Reaction time: 12 h.
Compounds 7 and 4 are identical in this case.
Other products — if at all detectable — were present in insignificant amounts.
This other product was bis-(4-anisyl)iodonium triflate (1H-NMR, MS), perhaps arising as per ref. 1.
The major other product was 1-naphthyl-4-nitrophenyl-iodonium triflate; the minor other product was bis-(4-anisyl)iodonium triflate (1H-NMR, MS).
A trace amount of 1-naphthyl-4-nitrophenyl-iodonium triflate, too small to be measured accurately, was apparent by 1H-NMR and MS.
