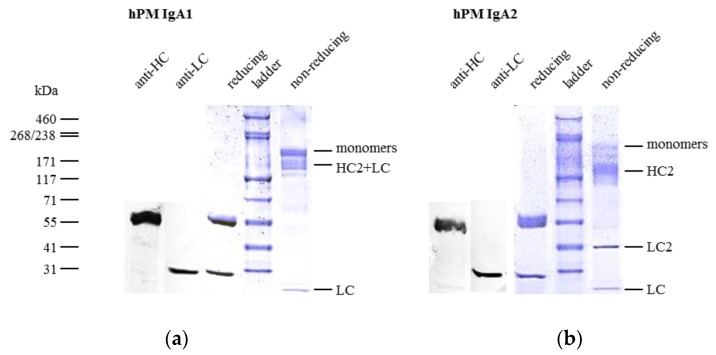
Figure 3.
Purity assessment and comparison of affinity purified IgA1 (a) and IgA2 (b) by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blots. Antibodies were analyzed using 4–15% gradient gels; in reducing conditions, only bands corresponding to the size of heavy chain (HC) and light chain (LC) were detected. Identities of bands in reducing conditions were confirmed by anti-HC and anti-LC Western blots (two left lanes). In non-reducing conditions, IgA1 antibodies showed a major band corresponding to monomers (HC2 + LC2) and minor bands for free LC and monomers lacking one LC (HC2 + LC). In contrast, IgA2 antibodies dissociated mainly in HC and LC dimers (HC2 and LC2, respectively) and LC. Bands with less intensity were detected, which corresponded to the size of IgA2 monomers or IgA2 monomers lacking one LC.