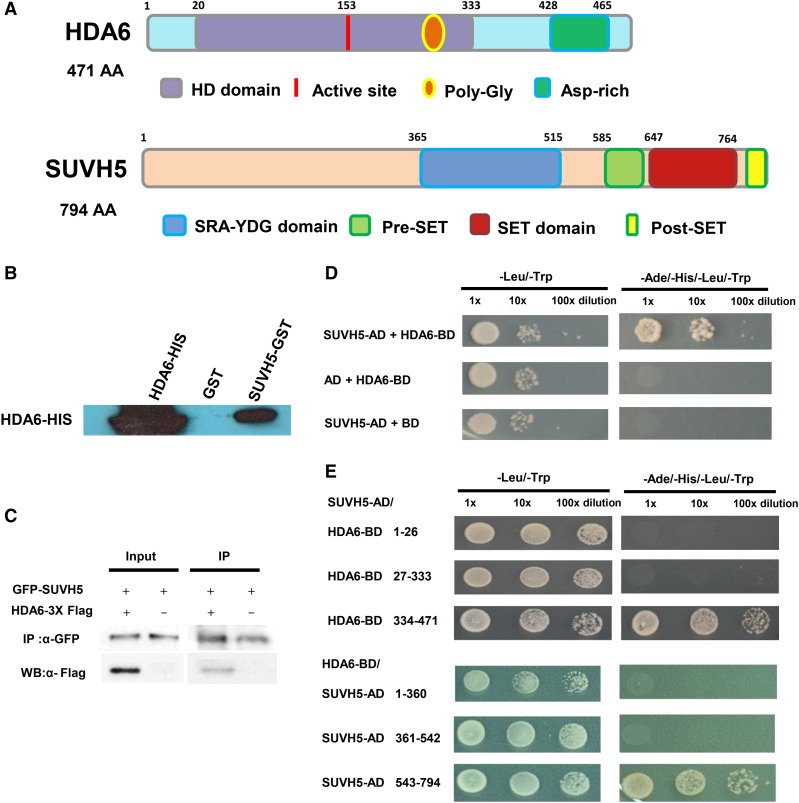
Figure 2.
Interaction of HDA6 and SUVH5 in in Vitro Pull-Down, Co-IP, and Yeast Two-Hybrid Assays.
(A) HDA6 and SUVH5 domain structures.
(B) SUVH5 interacts with HDA6 in in vitro pull-down assays. GST-SUVH5 or GST was incubated with HDA6-His and GST affinity resins, and the bound proteins were eluted from the resins and probed with the anti-His antibody.
(C) Interaction of HDA6 and SUVH5 in coimmunoprecipitation assays. Protein extracts coexpressing pro35S:GFP-SUVH5 and pro35S:HDA6-3X FLAG in N. benthamiana leaves were immunoprecipitated using anti-GFP antibody and analyzed by protein gel blot analysis.
(D) Yeast two-hybrid assays showing that full-length SUVH5 interacts with full-length HDA6.
(E) The C-terminal regions of HDA6 (334–471 amino acids) and SUVH5 (543–794 amino acids with Pre-SET and SET domains) are responsible for the protein-protein interactions.