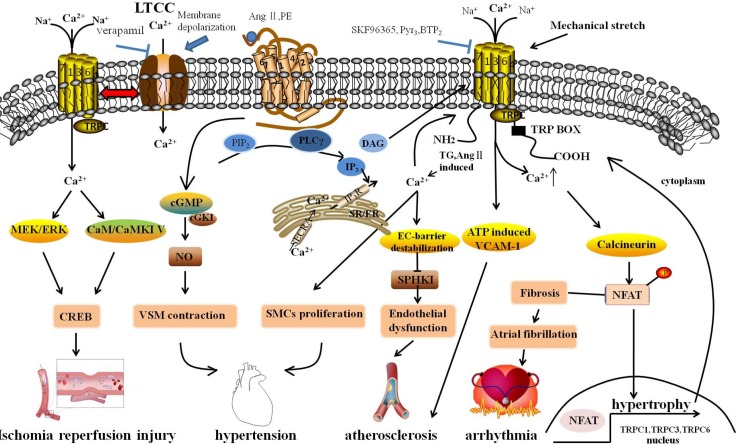
Fig. 1.
Molecular mechanism underlying cardiovascular diseases associated with the changing of intracellular Ca2+ through TRPCs. GPCRs, releasing DAG and IP3 via PIP2 with the subsequent activation of PLC, were stimulated by Ang II and PE, which were hypertrophic stimuli. DAG stimulated ROCs, including TRPC3 and TRPC6, resulting in extracellular Ca2+ influx. IP3 activated SOCE in response to depletion of intracellular Ca2+ stores by Ca2+ release in the SR/ER and subsequently activated TRPCs. The sustained TRPC-mediated Ca2+ entry directly activated the calcineurin-NFAT pathway, subsequently resulting in the activation of hypertrophic gene expression, including TRPC1, TRPC3 and TRPC6. Simultaneously, after activating, NFAT might activate TRPC gene expression through a positive feedback mechanism. TRPCs interacted with the LTCC through membrane depolarization, playing a role in regulation of cardiac pacemaking, conduction, ventricular activity, and contractility. Mechanical stretch caused arrhythmia through the activation of SACs to elevate cytosolic Ca2+ levels. Fibroblast regulated by Ca2+-permeable TRPCs might be associated with AF, and fibroblast proliferation and differentiation are a central feature in AF-promoting remodeling. TRPCs maintained adherens junction plasticity and enabled EC-barrier destabilization by suppressing SPHK1 expression to induce endothelial hyperpermeability, leading to atherosclerosis. In addition, the omission of extracellular Ca2+ with channel blockers (SKF96365, Pyr3) reduced monocyte adhesion and ATP-induced VCAM-1 and also relieved the progress of atherosclerosis. The rise of cytosolic [Ca2+]i promoted SMC proliferation. TRPC channels associated with vascular remodeling caused hyperplasia of SMCs. Moreover, TRPCs participated in blood pressure regulation due to receptor-mediated and pressure-induced changes in VSMC cytosolic Ca2+. Signaling via cGKI in vascular smooth muscle, by which endothelial NO regulated vascular tone, caused VSMC contraction. Activated TRPCs can activate downstream effectors and CREB proteins that have many physiological functions; TRPCs activated in neurons are linked to numerous stimuli, including growth factors, hormones, and neuronal activity through the Ras/MEK/ERK and CaM/CaMKIV pathways. GPCRs, G protein-coupled receptor; Ang II, Angiotensin II; PE, phenylephrine; ROCs, receptor-operated channels; SOCE, store-operated Ca2+ entry; LTCC, L-type voltage-gated calcium channel; SACs, stretch-activated ion channels; AF, atrial fibrillation; SPHK1, sphingosine kinase 1; VCAM-1, Vascular cell adhesion molecule-1; SMCs, smooth muscle cells; VSMC, vascular smooth muscle cells; cGKI, cGMP-dependent protein kinase I; CREB, cAMP/Ca2+-response element-binding.