Figure 1. Dual-fluorescent Reporters for Translational Errors.
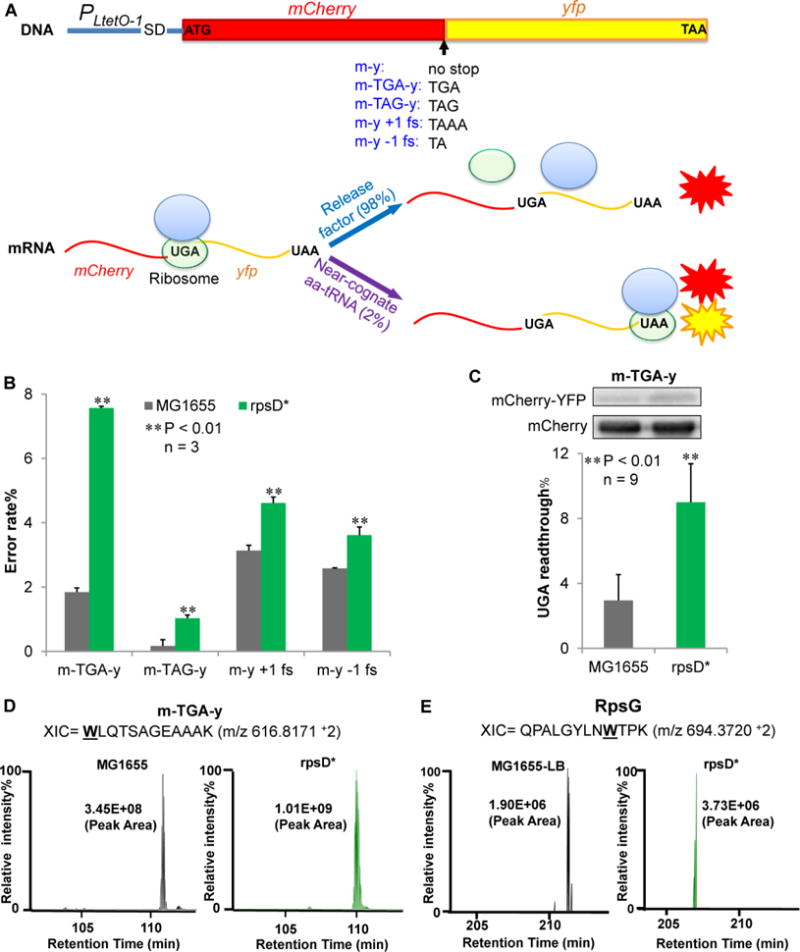
(A) Dual-fluorescence reporters that measure translational errors. MCherry (m) and yfp (y) genes are fused under the control of a constitutive promoter PLtetO-1. At the end of mCherry is a stop codon, a frameshifting (fs) codon, or no stop codon. Readthrough of the stop codon or frameshifting produces a single mCherry-YFP fusion protein and yields YFP signal. (B) The reporters on a low-copy number plasmid were expressed for 24 hours in Luria-Bertani broth (LB) at 37 °C in wild-type (MG1655) and ribosomal error-prone (rpsD*) E. coli strains. Fluorescence was quantified by spectrometry on a plate reader. The error rate was calculated as the YFP/mCherry ratio of the error reporter normalized by the YFP/mCherry ratio of the m-y control. (C) Western blotting showing that UGA readthrough of the m-TGA-y reporter. Anti-mCherry antibody was used to detect both mCherry-YFP fusion and mCherry. Data are represented as mean ± standard deviation. (D, E) Label-free quantitative mass spectrometry analysis of UGA readthrough in the dual-fluorescence reporter protein (m-TGA-y) and native protein RpsG. Extracted ion chromatograms (XIC) for the m-TGA-y reporter peptide WLQTSAGEAAAK (W, or tryptophan, marks the UGA readthrough site in the peptide) and the RpsG peptide QPALGYLNWTPK are shown from two different strains. XIC peak area indicates the relative abundance of the detected peptide. Relative peak intensities were fixed at 100% individually for comparative purposes.
