Short summary
Cow’s milk allergy (CMA) is increasing in prevalence, affecting approximately 4% of children. Cow’s milk (CM) is a common cause of fatal/ near fatal anaphylactic reactions. Understanding of CM-specific CD4+ T-cells responses to milk allergens should help elucidate the pathological mechanisms of persistent CMA. Milk allergen epitopes specific T-cells were examined in CMA subjects. Frequencies and phenotypes of these T-cells were found to be different between older and younger subjects.
Keywords: Food allergy, Cow’s milk allergy, Bos d, αS1-casein, T-cells, MHC class II tetramers, epitopes
To the Editor
Cow’s Milk Allergy (CMA) is the most common food allergy in children(1), approximately 42% of children can outgrow their CMA by 8 years of age(1). Whey (β-lactoglobulin the most abundant) and casein proteins are the major milk allergens (2). The composite allergen casein consists of several isoforms: αS1-casein, αS2-casein, β-casein and κ-casein(3). IgE sensitization is particularly frequent against αS1-casein, inducing strong immediate or delayed allergic reactions (4). Previous studies have shown that patients with persistent CMA showed IgE reactivity to epitopes from the casein group, specifically to αS1-casein, as compared to patients who developed clinical tolerance (5). Thus, we hypothesized that patients with persistent, IgE mediated CMA may have abnormal T-cell responses to casein. Additionally, little is known about specific T-cell responses toward these allergens in adults and children with CMA and non-allergic subjects.
A total of 26 allergic subjects, including 18 CM allergic subjects >8 years of age and 8 CM allergic subjects ≤8 years of age with persistent CMA (including subjects that are tolerant to baked milk products (n=8)) were recruited for this study (see Methods section in this article’s Online Repository at www.jacionline.org). Characteristics of the recruited milk-allergic subjects are shown in Table E1. 18 HLA-matched subjects including 13 non-atopic control subjects, and 5 patients with peanut or walnut allergy without milk allergy were recruited as control subjects (Table E1). Tetramer-guided epitope mapping approach was utilized to identify Bos d-specific-CD4+ T-cell epitopes. A total of 23 epitopes were identified: 3 β-lactoglobulin-, 5 αS1-casein-(including 4 previously identified(6)), 3 αS2-casein-, 8 β-casein- and 4 κ-casein-T-cell epitopes (see Fig E1 and Table E2 for HLA-restriction information and epitope specificity between cohorts in this article’s Online Repository at www.jacionline.org). Seven of the current identified epitopes have been previously reported in IEDB.
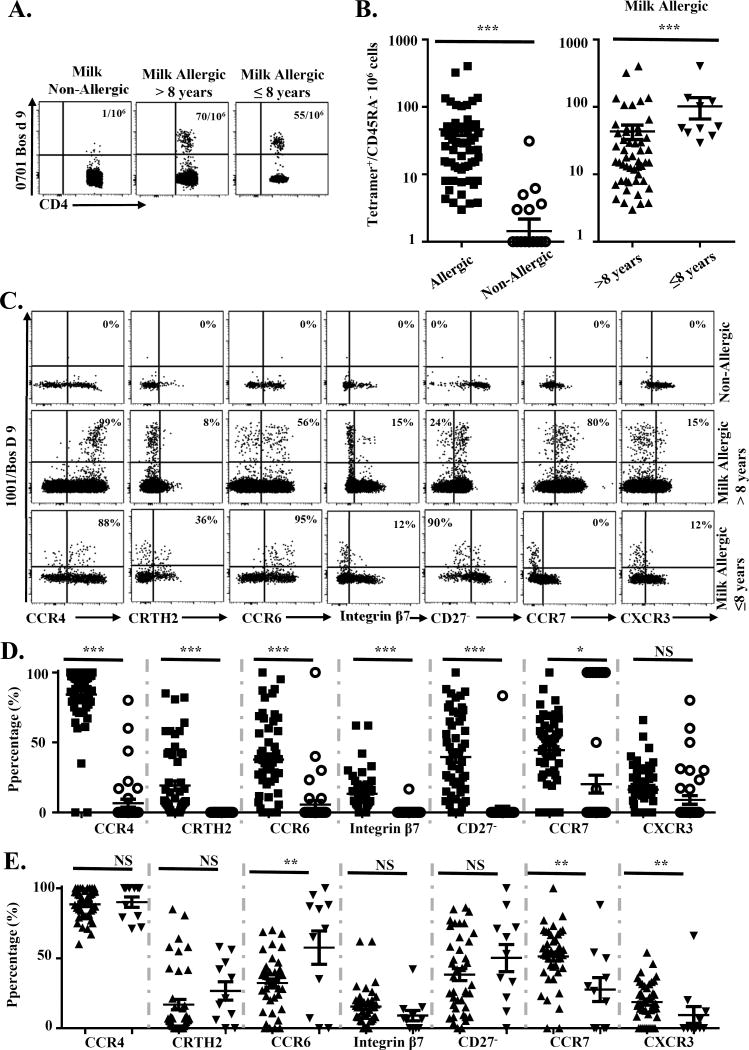
The anti-phycoerythrin (PE) magnetic bead tetramer enrichment approach was utilized to determine the frequencies of Bos d-specific T cells ex vivo. Bos d epitope-specific memory (CD45RA−) CD4+T-cells were detected in high frequencies in allergic subjects, while lower frequencies were observed in non-allergic subjects (Fig 1A and 1B). A significant difference in frequencies of Bos d-specific cells between CM allergic subjects >8 years of age and children ≤8 years of age with CMA was observed (Fig 1A and B). Regarding each allergen individually, β-lactoglobulin, αS1-casein, αS2-casein, β-casein and κ-casein-epitope specific memory T cells responses were detected in 6/23(26%), 13/23(57%), 7/23(30%), 11/23(48%) and 4/23(17%) of CMA subjects, while responses for subjects ≤8 years of age were 0/7 (0%), 4/7(57%), 1/7(14%), 3/7(42%) and 0/7(0%) and for subjects >8 years were 6/16(38%), 9/16(56%), 6/16(38%), 8/16(50%) and 4/16(25%) respectively. Responses for subjects with baked milk tolerance were 3/7(43%), 4/7(57%), 3/7(43%), 2/7(29%) and 1/7(14%) and for subjects with baked milk intolerance were 3/16(19%), 9/16(56%), 4/16(25%), 9/16(56%) and 3/16(19%) respectively. These results show that αS1-casein and β-casein responses were the most prevalent Bos d-specific T cell responses in CMA subjects and suggest a possibility of epitope spreading in older subjects as well as differential allergen recognition between the baked milk sensitized and the baked milk tolerance subjects. CD154 upregulation assay was also used to examine the overall T-cell responses towards these allergens in 8 of 26 milk allergic subjects. Strong αS1-casein-specific responses were also observed, confirming our MHC class II tetramer staining (see Fig E2 in this article’s Online Repository at www.jacionline.org). In total, these results suggested that majority of milk-specific CD4+ T cell responses in CMA subjects were directed against αS1-casein and β-casein. The current data is also in agreement with a previous study that showed frequency of casein-specific T cells was higher in CMA compared to non-CMA children(7).
Figure 1. Frequency and phenotype of Bos d-epitope-specific CD4+ T-cells.
A, Frequencies of Bos d-epitope-specific CD45RA−CD4+T-cells in subjects with and without CMA. B, Comparison of Bos d-specific CD4+T-cell frequency for each epitope described (data point) between milk allergic (n=23; filled squares) and non-allergic subjects (n=13; opened circles); and adults, teenagers and children >8 years old (n=16; triangles) and children ≤8 years old (n=7; downside triangles) with CMA. C, PBMC from a non-allergic and allergic subjects were stained with PE-labeled DRB1*10:01/Bos d 9145-164 tetramers and a panel of antibodies. The percentages of memory Bos d-specific T cells that expressed the surface marker of interest are as indicated. D, Phenotype of Bos d-specific T-cells in allergic and non-allergic subjects and in E. CMA >8 years old and CMA children ≤8 years old. Symbols in D and E are identical to those used in B. A Student t test was used in the statistical analysis. *P<0.05, **P<0.01, ***P<0.001, NS. Not significant.
Chemokine receptor and differentiation marker expression of Bos d-specific T-cells was analyzed by ex vivo tetramer staining of PBMC (Fig 1C, 1D and 1E). Detectable Bos d-specific CD4+T-cells in allergic subjects were CCR4+, suggesting a TH2 phenotype. In addition, CRTH2+ and CD27− T cells were observed (Fig 1C and 1D), which indicates a terminally differentiated TH2 phenotype(8). CCR4+ T cells that co-expressed CCR6 and Integrin β7 were also observed in subjects with CMA and were more common in children ≤8 years of age than older subjects with persistent CMA, suggesting that a sub-population of these cells account for a TH17-like response (Fig E3A & B). CCR6+CRTH2+ T-cells were detected but to a lesser degree (Fig E3C and D). However, we did not observe differences between allergic adults and children for these populations. On the other hand, higher CCR7 and CXCR3 expression (Fig 1C and 1E) were observed in subjects >8 years of age. The presence of more CXCR3+ and CCR7+ milk specific cells imply TH1 and TCM (central memory T cells as defined by expression of CD27 and CCR7) milk specific cells are more common in CM allergic subjects >8 years of age than in children ≤8 years of age with CMA. .No difference in frequencies and phenotypes amongst subjects with baked milk tolerance or intolerance was observed (Data not shown).
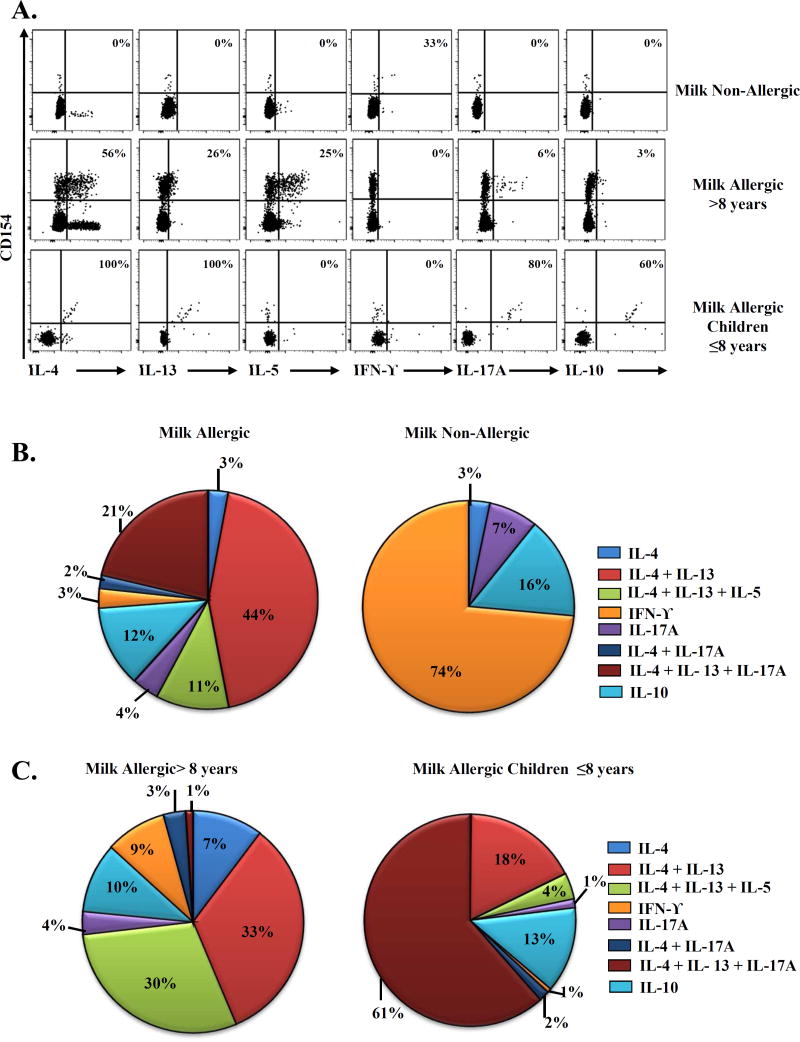
The CD154 upregulation assay was used to analyze the cytokine profiles of Bos d specific T-cells (Fig 2A). In subjects with CMA, a dominant TH2 response was observed, which accounted for 58% of the overall response (Fig 2B). Amongst the TH2 cytokine producers, cells can be classified into IL-4 producers (3%), IL-4/IL-13 (44%) producers, and IL-4/IL-5/IL-13 (11%) producers. We did not detect cells that produced IL-5 or IL-13 alone. Interestingly, a subdominant TH2/TH17 response was also observed, which accounted for 27%% of the overall T-cell response (Fig 2B). Amongst IL-17A producers, cells can be classified into IL-17A producers (4%), IL-4/IL-17A producers (2%) and IL-4/IL-13/IL-17A producers (21%). Interestingly, higher proportions of IL-4/IL-5/IL-13 producers (30%) and IL-4 producers (33%) were detected in CM allergic subjects >8 years of age (Fig 2C), in contrast higher proportions of IL-4/IL-13/IL-17A producers (61%) were detected in children ≤8 years of age (Fig 2C). There was no difference in the cytokines profiles of baked milk tolerant and intolerant subjects (Data not shown). Conversely, T-cells from non-allergic subjects produced IFN-γ, IL-10, or both, with low IL-17A (Fig 2A and 2B). These results confirmed the observed TH2 and TH2/TH17 phenotypes in our ex vivo experiments and our previous studies that these phenotypes could be a potential trademark in food allergy(8).
Figure 2. Cytokine profiles of Bos d-reactive T-cells.
A, First row, Cytokine profile in a DRB1*01:01 non-allergic subject. Second row, Cytokine profile in a DRB1*01:01 allergic adult. Third row, Cytokine profile in a DRB1*01:01 allergic child. The percentages of memory CD154+ Bos d-reactive cytokine producing T-cells are as indicated. B, and C, Cytokine profiles of Bos d-reactive T-cells in non-allergic (n=13) and allergic subjects (n=21), and between adults, teenagers and children >8 (n=14) and children ≤ 8 years of age (n=7) with CMA. Data are presented as the mean frequency of cytokine producing T-cells from each group in pie charts.
The current study implicates an important role of Bos d-specific T-cell responses in the persistence of CMA. In older children and adults with CMA, a committed TH2 response was observed (Fig. 2B, IL4/IL5/IL13 triple cytokine producers). On the other hand, in younger children with CMA, TH2/TH17 responses were more prevalent, suggesting that these T-cell populations are not fully committed into the TH2 phenotype and could explain loss of CMA in younger children. Moreover, TCM are less susceptible to deletion by allergen specific immunotherapy in a murine model(9). Accumulation of CCR7+CD27+ Bos d-epitope-specific T cells (TCM) in adults might be indicative of CMA persistence and also complicate possible oral immunotherapy for CMA. Knowledge of CM-epitope-specific T-cell responses will be useful in devising novel strategies to halt and reverse the progression of CMA.
Supplementary Material
Acknowledgments
Supported by US National Institutes of Health contract HHSN272200700046c.
We thank Kavitha Gilroy and Sylvia Posso for help with subject recruitment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Discolure of potential conflict of interest: L.D. Archila, W.W. Kwok have received research support from the US National Institutes of Health. The rest of authors declare that they have no relevant conflict of interest.
References
- 1.Spergel JM. Natural history of cow's milk allergy. J Allergy Clin Immunol. 2013;131(3):813–4. doi: 10.1016/j.jaci.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2007;120(5):1172–7. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Wal JM. Cow's milk allergens. Allergy. 1998;53(11):1013–22. doi: 10.1111/j.1398-9995.1998.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 4.Fiocchi A, Bouygue GR, Albarini M, Restani P. Molecular diagnosis of cow's milk allergy. Curr Opin Allergy Clin Immunol. 2011;11(3):216–21. doi: 10.1097/ACI.0b013e32834694ef. [DOI] [PubMed] [Google Scholar]
- 5.Vila L, Beyer K, Jarvinen KM, Chatchatee P, Bardina L, Sampson HA. Role of conformational and linear epitopes in the achievement of tolerance in cow's milk allergy. Clin Exp Allergy. 2001;31(10):1599–606. doi: 10.1046/j.1365-2222.2001.01218.x. [DOI] [PubMed] [Google Scholar]
- 6.Elsayed S, Eriksen J, Oysaed LK, Idsoe R, Hill DJ. T cell recognition pattern of bovine milk alphaS1-casein and its peptides. Mol Immunol. 2004;41(12):1225–34. doi: 10.1016/j.molimm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Michaud B, Aroulandom J, Baiz N, Amat F, Gouvis-Echraghi R, Candon S, et al. Casein-specific IL-4- and IL-13-secreting T cells: a tool to implement diagnosis of cow's milk allergy. Allergy. 2014;69(11):1473–80. doi: 10.1111/all.12484. [DOI] [PubMed] [Google Scholar]
- 8.Archila LD, Jeong D, Pascal M, Bartra J, Juan M, Robinson D, et al. Jug r 2-reactive CD4 T cells have a dominant immune role in walnut allergy. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackenzie KJ, Nowakowska DJ, Leech MD, McFarlane AJ, Wilson C, Fitch PM, et al. Effector and central memory T helper 2 cells respond differently to peptide immunotherapy. Proc Natl Acad Sci U S A. 2014;111(8):E784–E793. doi: 10.1073/pnas.1316178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.