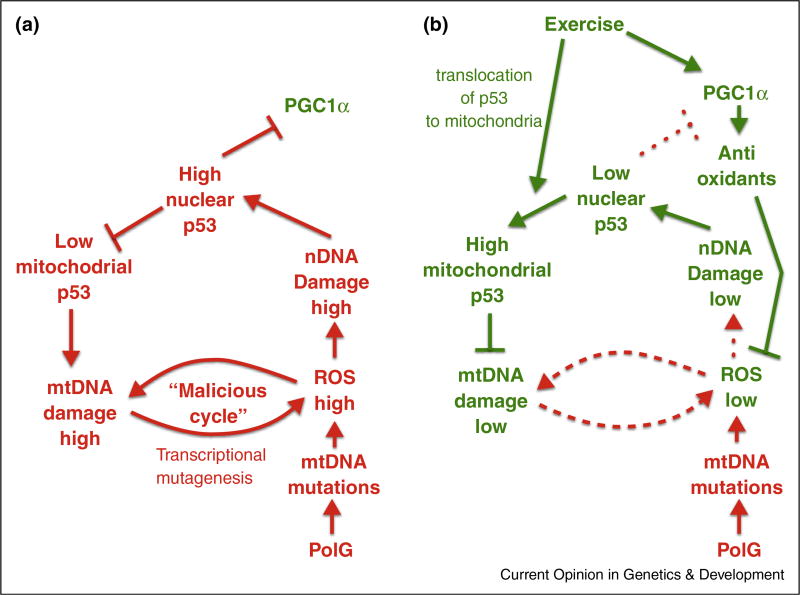
Figure 2.
Hypothetical mechanism underpinning the amelioration of premature aging in the mtDNA mutator mouse by endurance exercise. (a) Sedentary PolG mouse: ‘a double cycle’. Defective polymerase gamma (bottom) creates an influx of de novo mutations in mtDNA. The accumulating mtDNA mutations cause increase of ROS production, which leads to increased oxidative mtDNA and nuclear DNA damage. mtDNA damage adds to the ROS production caused by mtDNA mutations, presumably via transcription mutagenesis, which completes the hypothetical ‘malicious cycle’. Moreover, nuclear DNA damage triggers the translocation of the p53 into the nucleus, which reduces the available pool of p53 for mitochondria. The paucity of p53, a likely DNA repair enzyme, results in an even higher level of DNA damage, resulting in another self-sustaining cycle. (b) Exercised PolG mouse: Exercise intercepts the ‘double cycle’ in two ways: First, exercise promotes the expression of PGC-1α, which in turn activates an antioxidant gene expression network. This serves to attenuate overall oxidative stress and nuclear DNA damage, and results in the subsequent release of p53 from the nucleus, making the p53 pool available for the mitochondria. PGC-1α upregulation also results in an increased mitochondrial biogenesis. Second, the translocation of p53 into mitochondria, where p53 serves as a repair enzyme, reduces the mtDNA damage and thus disrupts the ‘malicious cycle’. This ultimately sets the system in a different equilibrium with lower mtDNA damage.