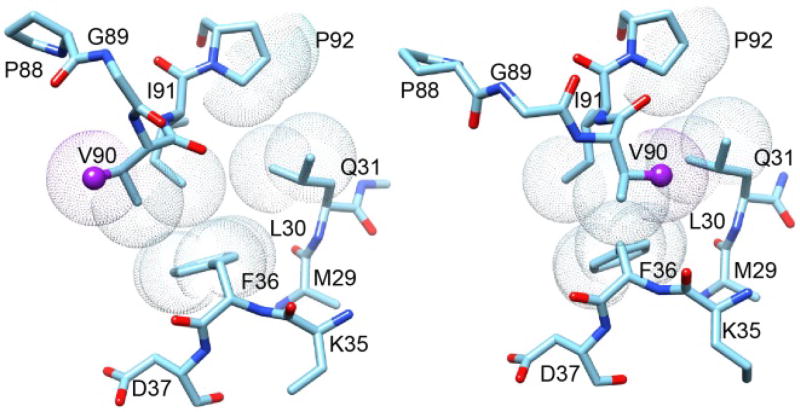
Fig. 13. Superposition of region surrounding the Gly 89–Val 90 peptide bond from the two crystal forms of FKBP12.6.
This region of the P3121 crystal form structure of cysteine-free FKBP12.6 is illustrated on the left. The Cγ of Val 90 (sphere) is pointed toward the catalytic cleft as is typical of analogous crystal structures for FKBP12. On the right is displayed this region from the P21 crystal form structure in which the Val 90 Cγ is pointed away from the catalytic cleft. The difference in conformation primarily arises from the peptide linkage between Gly 89 and Val 90 being flipped in the P21 crystal form relative to its position in the P3121 crystal form. Van der Waals surfaces are illustrated for the evolutionarily conserved hydrophobic sidechain interactions between the β2 and β3a strands and the tip of the β4–β5 loop.