Figure 1.
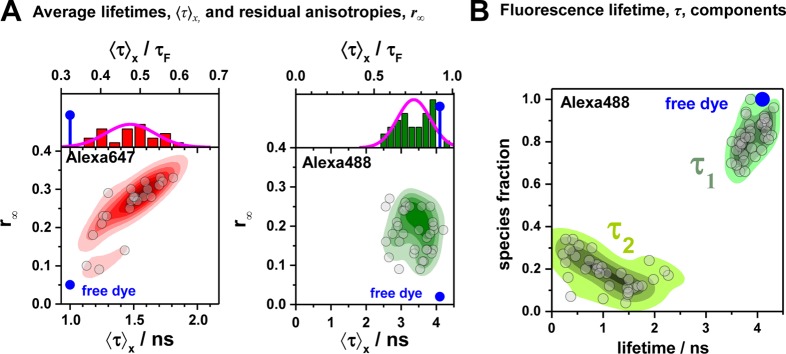
Fluorescence properties of the dyes Alexa488 and Alexa647 tethered to proteins are sample-dependent due to variations of the local dye environment. Average fluorescence lifetimes, ⟨τ⟩x, and residual anisotropies, r∞, of the fluorophores Alexa647 and Alexa488 attached via maleimide or hydroxylamine click chemistry to different amino acids of various proteins (human guanylate binding protein 1, T4 lysozyme, postsynaptic density protein 95, lipase foldase of Pseudomonas aeruginosa and the cyclin-dependent kinase inhibitor 1B). (A) For each sample, the species weighted averaged lifetime ⟨τ⟩x and r∞ are shown as dots overlaid by a Gaussian kernel density estimation.56 The fluorescence parameters are compiled in Table S1 for Alexa647 and in Table S2 for Alexa488 together with individual fluorescence lifetimes from a detailed decay analysis. Using radiative lifetimes of τF = 3.1 ns and τF = 4.5 ns for Alexa64757 and Alexa488, respectively, the relative brightnesses, ⟨τ⟩x/τF, were calculated. The average values of all Alexa647 and Alexa488 samples are ⟨τ⟩x/τF = 0.43 ± 0.07 and ⟨τ⟩x/τF = 0.76 ± 0.11, respectively. The average residual anisotropies of Alexa647 and Alexa488 for all samples are ⟨r∞⟩ = 0.25 ± 0.07 and ⟨r∞⟩ = 0.18 ± 0.05, respectively. (B) The fluorescence intensity decays of the Alexa488 samples were formally resolved into two components τ1 and τ2 with the respective fractions x1 and x2 = 1 – x1. For each sample the lifetimes and fractions are shown as open circles overlaid with a Gaussian-kernel density estimation (green). The average lifetimes of the populations are τ1 = 3.9 ± 0.2 ns and τ2 = 1.0 ± 0.5 ns with species fractions of x1 = 0.8 ± 0.1 and x2 = 0.2 ± 0.1, respectively. The presented data are summarized in Table S2.