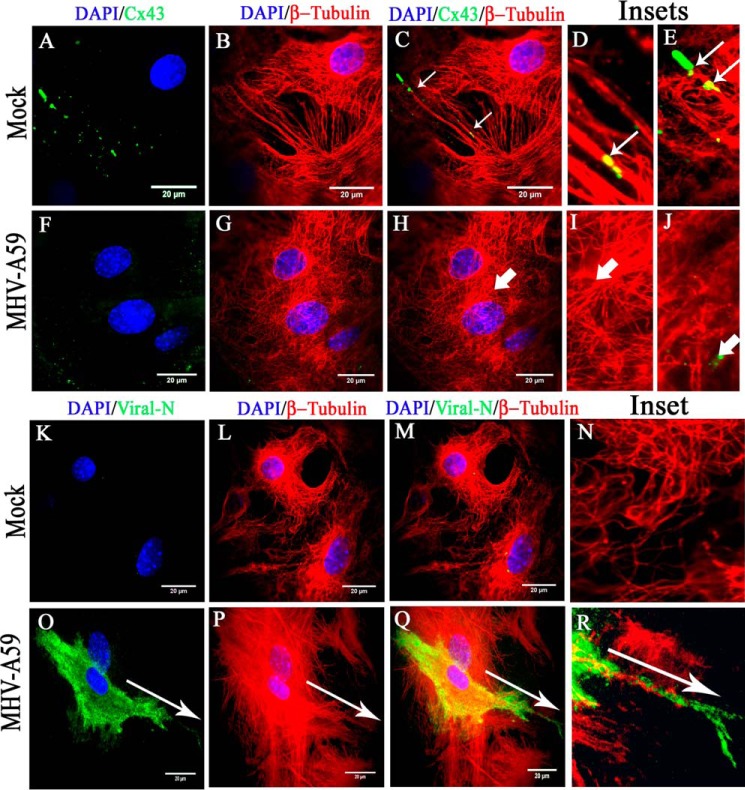
Figure 5.
TIRF microscopy confirmed association of MT network with cortical Cx43, which was depleted due to MT/MHV-A59 interaction. Primary astrocytes, plated on glass coverslips, were mock-infected or infected with MHV-A59 and, upon staining with β-tubulin (red) and Cx43 (green), subjected to TIRF imaging to specifically capture the immunofluorescent signal of cortical Cx43. The imaging depth was limited to within 100 nm of the coverslip. Simultaneously, the whole-cell MT network was imaged by epifluorescence microscopy. In control astrocytes, Cx43 (A) was observed to be closely associated with positive ends of MTs or the MT network (B and C (thin arrow)). Insets show that Cx43 molecules were either precisely aligned along the MT thread (D, thin arrow) or present at the tip of the MT thread (E, thin arrow). In MHV-A59–infected cells, minimal cell surface-associated Cx43 signal was observed (F), because intracellular compartment–retained Cx43 was restricted from reaching the cell surface. MT morphology appeared normal (G). As expected, in infected cells, Cx43 molecules were not associated with positive ends of MTs (H, I, and J, thick arrow) Similarly, mock- and virus-infected cultures of primary astrocytes were stained for β-tubulin (red) and viral N (green), and TIRF imaging was performed as described before. Mock-infected astrocytes showed no viral N–specific signal (K and M), and MT morphology was normal (L and M). The inset shows a digitally magnified area of the mock-infected cell's periphery (N). MHV-A59–infected astrocyte cultures showed the presence of viral N staining (O and Q), and MT morphology is shown in P. Merged images show a pattern of viral spread at the cell surface in a single infected cell, where viral N staining colocalized with MTs (Q). The inset shows that the viral particles were aligned along the MT thread at the cell surface (R). The arrow shows the alignment of viral spread in O–R.