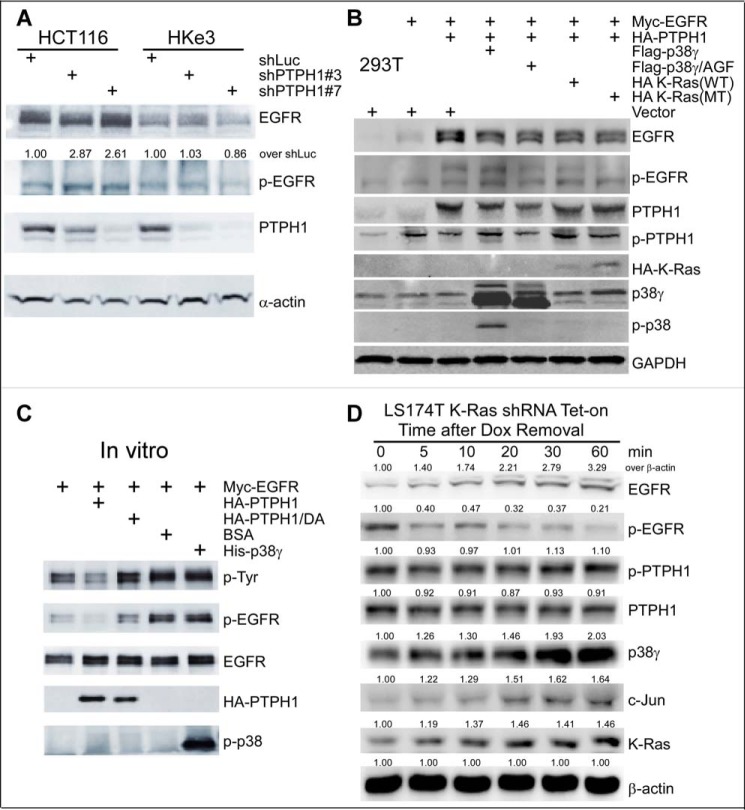
Figure 5.
PTPH1 is only active in decreasing EGFR tyrosine phosphorylation in K-Ras mutant cells and cooperates with mutant K-Ras and p38γ to promote EGFR dephosphorylation. A, control cells and cells stably depleted of PTPH1 were analyzed by WB for EGFR expression and phosphorylation (EGFR/Tyr-1173); relative levels of p-EGFR in shPTPH1 versus shLuc cells were normalized to EGFR (55). B, the indicated constructs were transiently transfected into 293T cells, and the resultant cells were analyzed by WB. C, Myc-EGFR protein isolated from transiently transfected 293T cells was incubated in vitro with the indicated proteins, and the mixtures were then analyzed by WB for p-EGFR levels. D, K-Ras mutation in situ activates the p38γ/c-Jun/PTPH1 signaling network, leading to both increased EGFR protein expression and decreased EGFR/Tyr-1173 phosphorylation. Cells were cultured with Dox for 72 h to silence the MT K-Ras. Protein lysates were prepared at the indicated time after Dox removal for K-Ras re-expression and analyzed by WB. The number indicates relative protein amounts over β-actin at each time point (normalized to 0 min), and similar results were obtained in a separate experiment.