Abstract
Staphylococcus aureus is a Gram-positive bacterial pathogen that produces a range of infections including cellulitis, pneumonia, and septicemia. The principle mechanism in antistaphylococcal host defense is opsonization with antibodies and complement proteins, followed by phagocytic clearance. Here we use a previously developed technique for installing chemical epitopes in the peptidoglycan cell wall to show that surface glycopolymers known as wall teichoic acids conceal cell wall epitopes, preventing their recognition and opsonization by antibodies. Thus, our results reveal a previously unrecognized immunoevasive role for wall teichoic acids in S. aureus: repulsion of peptidoglycan-targeted antibodies
Graphical abstract
Staphylococcus aureus is a Gram-positive pathogen that claims nearly 20 000 lives in the United States each year through serious infections such as pneumonia and septicemia and imposes a major financial burden through common infections such as cellulitis.1 Host defense against S. aureus primarily depends on phagocytic clearance by macrophages and neutrophils recruited to sites of infection. These innate immune cells identify and phagocytose S. aureus via opsonins that coat the bacterial cell surface— principally, antibodies and complement.2 Following internalization, bacteria are killed through multiple mechanisms, including oxidative damage, enzymatic degradation, and antimicrobial peptide-induced lysis.2 S. aureus has evolved a myriad of immunoevasion mechanisms targeting each of these steps: i.e., phagocyte recruitment, immune detection, and intraphagosomal killing.3
Wall teichoic acids (WTA) are glycopolymers covalently attached to the peptidoglycan (PG) cell wall in Gram-positive bacteria. Extending beyond the cell wall, they form a dense, highly charged glycan layer at the cell surface,4,5 where they play key roles in S. aureus physiology, regulating ion homeostasis6, autolysis7–10, and PG biosynthesis11,12. WTAs also contribute to the pathogenesis of S. aureus infection by mediating adherence to host tissues.13–16 In addition, the WTA layer serves as a physical barrier to shield S. aureus from a broad array of environmental threats, ranging from antimicrobial surfactants to bacteriolytic enzymes (e.g., lysozyme).17,18
In keeping with this protective role, we hypothesized that WTAs may also function as an “immunological cloak” for S. aureus, preventing antibodies from recognizing and opsonizing the cell wall. PG represents an ideal target for the immune system because its structure is highly conserved across bacterial species and absent in mammalian tissue.19 Indeed, antibodies targeting the cell wall could conceivably provide broad protection against S. aureus (and possibly other Gram-positive species as well), given the conservation of PG structure and the fact that Gram-positive bacteria lack an outer membrane to protect PG from immune recognition. Thus, concealing cell wall epitopes with WTAs would be a highly advantageous immunoevasive strategy.
To explore the hypothesis that WTAs block antibody binding to S. aureus PG, we first needed to identify a cell wall-specific antigen to which we could test recruitment of a cognate antibody. Unfortunately, commercially available antistaphylococcal PG antibodies are raised against whole cell immunogens (not purified20 or chemically synthesized21 PG antigens) and may therefore recognize non-PG epitopes. To circumvent this problem, we reasoned that we could install an exogenous chemical antigen in the cell wall and assess recruitment of a highly specific antibody to this foreign epitope.
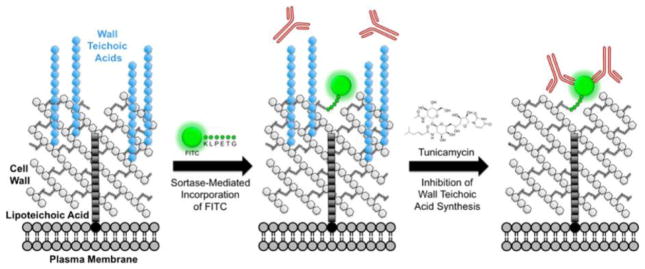
To execute this strategy, we made use of a “chemical surface display”22 technique previously developed in our lab, which enables insertion of nonnative chemical moieties into the cell wall of S. aureus via sortase A.23 Sortase A is an enzyme that resides on the extracellular face of the plasma membrane, where it recognizes the pentapeptide motif LPxTG in secreted proteins (wherein x can be any amino acid). The enzyme cleaves between the threonine and glycine of this motif and then attaches the LPxT peptide along with its N-terminal protein cargo to the PG bridge peptide (Figure S1a,b).24 Through the same mechanism, incubating bacteria with synthetic LPxTG peptides carrying N-terminal chemical cargo leads to covalent “biosynthetic” incorporation of exogenous compounds into the cell wall of S. aureus (Figure S1c).23 As shown previously, this labeling process takes place under standard laboratory growth conditions and has no appreciable effects on cell viability or morphology.23
Utilizing this technique, we sought to install the chemical epitope fluorescein isothiocyanate (FITC) into the cell wall of S. aureus using the probe K(FITC)-LPETG (Figure S1c). We then sought to assess recruitment of antibodies to PG-bound FITC in the presence and absence of WTAs in order to test the role of these glycopolymers in immunoevasion. Following the protocol of Campbell et al.,12 we depleted WTA by treating cells with 0.1 μg mL−1 tunicamycin, a concentration that selectively inhibits the first gene in the WTA biosynthetic pathway, TagO, without altering cell viability.12
To control for nonspecific sticking of the probe to the cell surface, which could lead to deposition of FITC epitopes on non-PG envelope structures (and thus “off-target” antibody recruiting), we labeled parallel sets of samples with the isomeric scrambled peptide K(FITC)-EGTLP. As demonstrated previously, this control peptide recapitulates the nonspecific binding properties of the LPETG probe but is not incorporated into PG by sortase A.23 Therefore, comparisons between EGTLP- and LPETG-treated samples allow for specific quantification of antibody recruitment to PG-bound epitopes.
As an additional control, we performed our experiments in the Δspa S. aureus Newman mutant. This strain lacks expression of protein A, a surface protein that binds to the Fc portion of many mammalian antibody isotypes.25 Hence, the use of Δspa S. aureus eliminates protein A-mediated antibody binding, allowing specific detection of antibody recruitment to FITC. To test whether Δspa S. aureus is capable of incorporating K(FITC)-LPETG, both in the presence and absence of WTA, we labeled mutant bacteria with and without tunicamycin. As shown in Figure S2a, flow cytometry measurements of cellular FITC fluorescence demonstrated effective labeling with K(FITC)-LPETG under both conditions.
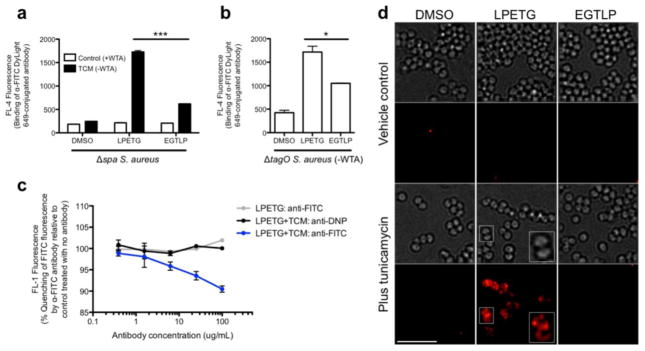
Having confirmed that the Δspa mutant efficiently incorporates FITC into the cell wall, we assessed antibody recruitment to PG-bound FITC by incubating cells with DyLight 649-conjugated anti-FITC antibodies, and then measuring cellular FL-4 fluorescence via flow cytometry. Strikingly, we observed no antibody binding at all in native bacteria, but after inhibition of WTA synthesis with tunicamycin, we observed substantial recruitment (Figure 1a).
Figure 1.
Antibodies are recruited to chemical epitopes within the S. aureus cell wall when WTAs are absent. (a) Δspa S. aureus Newman was labeled with K(FITC)-LPETG, K(FITC)-EGTLP, or DMSO vehicle control with or without 0.1 μg mL−1 tunicamycin and then incubated with 5 μg mL−1 DyLight 649-conjugated anti-FITC antibody. Antibody recruitment was assessed by measuring total cell FL-4 fluorescence (from the DyLight 649 fluorophore) via flow cytometry. (b) ΔtagO S. aureus Newman was labeled with peptides and analyzed for antibody recruitment by flow cytometry as in a. (c) Δspa S. aureus Newman was labeled with K(FITC)-LPETG with or without 0.1 μg mL−1 tunicamycin, and then incubated with anti-FITC antibodies. Fluorophore quenching was assessed by measuring total cell FL-1 fluorescence (from FITC) on a flow cytometer and expressed as a percentage of the fluorescence of unopsonized bacteria. Equimolar anti-DNP antibodies were used as a control. (d) Δspa S. aureus Newman was labeled with K(FITC)-LPETG, K(FITC)-EGTLP, or DMSO vehicle control with or without 0.1 μg mL−1 tunicamycin, and then incubated with DyLight 649-conjugated anti-FITC antibody. Widefield imaging was performed to visualize antibody localization. The magnified inset represents an image after deconvolution processing. Flow cytometry data shown are representative of multiple independent trials performed in technical duplicate; error bars indicate SEM. * denotes a P value <0.05. *** denotes a P value <0.001. Scale bar: 5 μm. Abbreviations: TCM, tunicamycin; WTA, wall teichoic acids.
The small increase in binding in the tunicamycin-treated EGTLP sample compared with the untreated EGTLP control most likely represents antibody recognition of probe that is nonspecifically adsorbed to the WTA-denuded cell wall. Indeed, flow cytometry quantification of EGTLP probe labeling demonstrated that the peptide bound much more avidly to cells in the absence of WTA (Figure S2b). This finding may be explained by the relative hydrophobicity of the exposed PG cell wall in TCM-treated cells compared to the highly charged WTA layer present in native bacteria—a property that likely promotes nonspecific sticking of peptide probes. However, as stated above, the substantial increase in antibody recruiting in the tunicamycin-treated LPETG sample compared to the corresponding EGTLP control demonstrates specific recognition of epitopes covalently linked to PG.
Next, to confirm that antibody binding to cell wall epitopes is a result of WTA removal per se and not an artifact of tunicamycin treatment, we tested recruitment in ΔtagO S. aureus Newman, which is genetically deficient in WTA synthesis (Figure 1b). In this strain, we did observe substantial binding of antibody to DMSO-treated cells (likely attributable to protein A-mediated capture) and to nonspecifically adsorbed EGTLP probe. However, there was also a significant increase in antibody recruitment to PG-bound FITC in ΔtagO S. aureus (Figure 1b) that was not observed in WTA-expressing cells (Figure 1a). This result further supports the essential role of WTAs in blocking antibody binding to cell wall epitopes.
We then leveraged a unique property of anti-FITC antibodies—their ability to quench the fluorescence of FITC upon binding—to demonstrate a direct physical interaction between antibody and PG-bound epitope. To this end, we labeled Δspa S. aureus with K(FITC)-LPETG in the presence and absence of tunicamycin and then incubated cells with various concentrations of anti-FITC antibodies. As shown in Figure 1c, there was a progressive decrease in FITC fluorescence with increasing concentrations of anti-FITC antibody in WTA-depleted S. aureus. This result indicates fluorescence quenching due to direct antibody binding to fluorophore. No such quenching was observed with an anti-DNP control, demonstrating specific antibody recognition of FITC. Quenching was also absent in WTA-expressing cells, again confirming WTA’s critical role in impeding antibody recruitment. Of note, the modest degree of quenching we observed (~10% at the highest antibody concentration) is likely attributable to the relative impermeability of the cell wall to antibodies. Binding is probably restricted to superficial PG-linked FITC molecules, whereas the majority of epitopes are likely buried within the PG meshwork, inaccessible to antibodies.
Imaging studies were then performed to further demonstrate that antibodies are able to recognize cell wall epitopes only in the absence of wall teichoic acids. First, we imaged recruitment of antibodies to FITC, and consistent with our flow cytometry results (Figure 1a), we found that antibodies only bound to cells that had been labeled with K(FITC)-LPETG and depleted of WTAs (Figure 1d).
Next, to directly visualize the relationship between epitope and antibody, we incorporated the more photostable fluorophore Alexa 488 into the cell wall via K(A488)-LPETG and recruited anti-A488 antibodies. As shown in Figure 2a (left panels), the A488 epitope was distributed uniformly throughout the cell wall, including the septum (see inset). Antibodies, meanwhile, only bound to the exterior portion of the cell wall, without penetrating into the septum. Super-resolution images further showed that antibody predominantly coated the surface of the cell wall (Figure 2b, Movie S1), a result that is fully consistent with the quenching data (Figure 1c), which suggested that only superficial PG-bound epitopes are available for binding.
Figure 2.
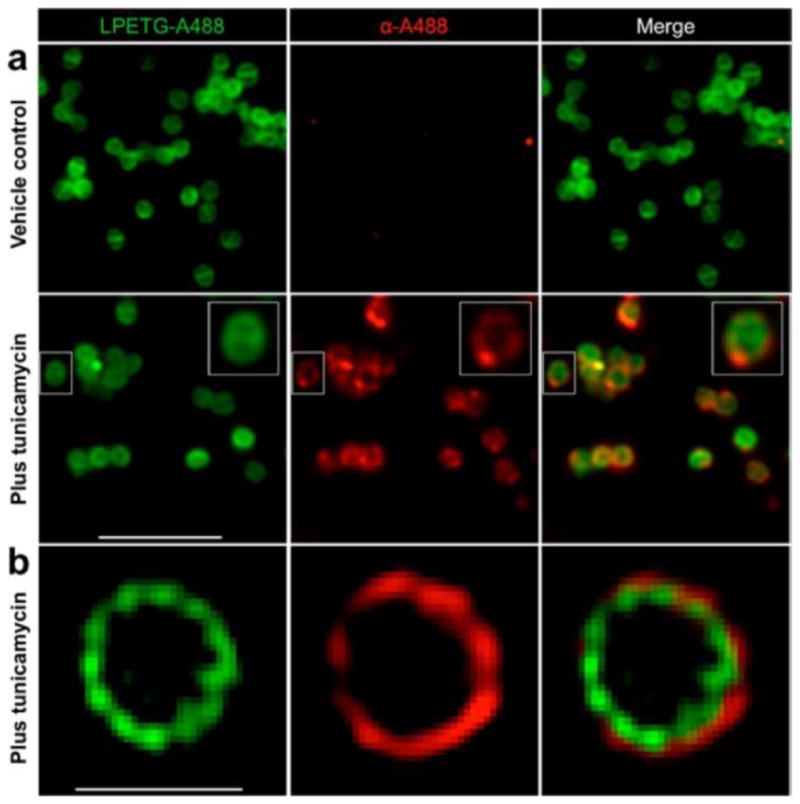
Antibodies bind predominantly to superficial PG-bound epitopes. Δspa S. aureus Newman was labeled with K(A488)-LPETG with or without 0.1 μg mL−1 tunicamycin and then incubated with anti-Alexa 488 primary antibodies, followed by Alexa 568-conjugated secondary antibodies. (a) Widefield imaging analysis was performed to visualize epitope and antibody localization. The magnified inset represents a dividing cell with peripherally located antibodies. (b) Structured illumination microscopy (SIM) imaging analysis was performed to visualize epitope and antibody localization at super resolution. Scale bar in (a): 5 μm. Scale bar in (b): 1 μm.
Finally, we sought to demonstrate that WTAs serve as a barrier against binding of not only antibodies but proteins in general. To this end, we tested whether the 53-kDa protein streptavidin could be recruited to PG-bound biotin in the presence and absence of WTAs in WT S. aureus (note: the use of the Δspa mutant in this experiment was unnecessary, as streptavidin does not bind to protein A). Indeed, consistent with the antibody recruiting results presented in Figure 1a, streptavidin only bound its target when WTA synthesis was inhibited (Figure 3).
Figure 3.
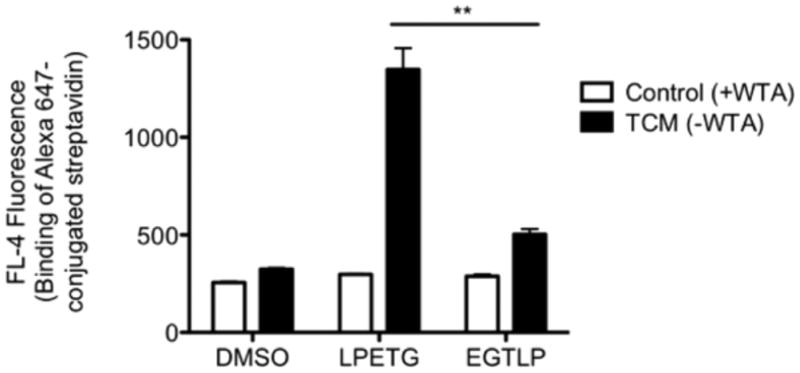
Streptavidin recruitment to PG-bound biotin requires WTA depletion. WT S. aureus Newman was labeled with K(biotin)-LPETG, K(biotin)-EGTLP, or DMSO control with or without 0.1 μg mL−1 tunicamycin and then incubated with Alexa 647-conjugated streptavidin. Protein recruitment was assessed by measuring total cell FL-4 fluorescence (from Alexa 647) on a flow cytometer. Experiment performed in technical duplicate; error bars indicate SEM. ** denotes a P value <0.01. Abbreviations: TCM, tunicamycin; WTA, wall teichoic acids.
In this study, we have employed a unique “chemical surface display”22 strategy to interrogate the role of WTA at the interface between S. aureus and the immune system. Using sortase-targeted peptides, we were able to biosynthetically incorporate several chemical epitopes (FITC, Alexa 488, and biotin) at a precisely defined site in the peptidoglycan sacculus under the WTA layer. Then, using specific recognition proteins for these epitopes, we generated multiple lines of evidence to show that WTAs serve as a barrier against opsonin recruitment to the cell wall (Figure 4). This barrier function most likely derives from the density and charge of WTAs, which in turn lead to steric and/or electrostatic repulsion of opsonins.
Figure 4.
Schematic of wall teichoic acids shielding cell wall epitopes from opsonins in S. aureus. K(FITC)-LPETG incorporated into the cell wall of S. aureus by sortase A is inaccessible to antibodies unless WTAs are depleted by tunicamycin treatment or by deletion of genes in the WTA biosynthetic pathway.
According to this model, cell wall-associated proteins (CWAPs, Figure S1) must extend beyond the WTA layer since they are recognized by antibodies in native S. aureus.26 This exposure is likely necessary for the particular functions of CWAPs (e.g., protein A intercepting host antibodies, clumping factor B mediating adhesion to host tissue, etc.27), but it also leaves them susceptible to antibody-mediated immunity. It may be that S. aureus is able to tolerate such immune responses because CWAPs are often functionally redundant and therefore poorly conserved.27 Indeed, vaccine studies have shown that broad protection against S. aureus cannot be achieved through an antibody response to any single CWAP; multiple antigens must be targeted simultaneously.28
In contrast, the basic structure of PG is nearly invariable between S. aureus strains. In fact, PG structure is so well-conserved that it represents a bacterial pathogen-associated molecular pattern (PAMP)—a molecular signature for the presence of invading bacteria—that is recognized by a dedicated set of innate immune receptors: NOD1 and NOD2.19 Thus, it would appear critical to conceal this highly conserved antigen from antibodies, which may otherwise provide broad, species-wide protection against S. aureus—and perhaps other Gram-positive bacteria as well.
It is interesting to note that antibodies against staphylococcal PG are virtually ubiquitous in human sera.29,30 The reason for their inefficacy against S. aureus is unclear, but it may be that they are simply obstructed from binding their targets by WTA. Indeed, the effectiveness of pharmacological WTA inhibitors and diminished virulence of WTA-depleted S. aureus in animal infection models may be attributable in part to the exposure of PG to such antibodies.13,15,16,31,32 Further studies will be needed to test this hypothesis.
WTA itself also serves as a target for the human immune system, as it is recognized by both antibodies and the soluble pattern recognition molecule, mannose binding lectin.33,34 Similarly to anti-PG immunity, humoral responses against WTA fail to provide adequate antistaphylococcal defense. However, murine vaccine studies have shown that boosting anti-WTA antibody titers can provide protection, suggesting that the inefficacy of WTA antibodies may be attributable to insufficient quantity rather than antigenic inaccessibility.35
The notion that WTA functions as a cloak for PG to prevent detection by the immune system fits well with several previous studies. Recently, Fura et al. reported an immunotherapeutic strategy against S. aureus in which dinitrophenol (DNP) modified D-amino acids are incorporated into peptidoglycan and used to recruit anti-DNP antibodies present in human serum.36 Consistent with our findings, they showed that antibody recruiting to PG-bound DNP greatly improved in the absence of WTA. Similarly, An et al. have demonstrated that human serum amyloid protein (SAP), a component of the acute phase response to infection, is able to recognize PG only in the absence of WTAs.37 Finally, in Drosophila, Atilano et al. have shown that WTAs prevent binding of the innate effector protein, peptidoglycan recognition protein (PGRP), to the staphylococcal cell wall.38 The same group recently built upon these findings, demonstrating that PGRP recognition of PG could be restored through deletion of the major autolysin, atl. They proposed that PG fibers projecting radially from the S. aureus cell wall (also observed in immunoelectron microscopy studies20) are able to extend beyond the WTA layer where they are susceptible to immune detection unless actively “trimmed” by atl.39 It should be noted, however, that WTA deletion per se disrupts atl localization8 and that deletion of WTA and atl have additive effects on PGRP recruitment;39 therefore the role of atl in PG exposure is likely multifactorial.
Overall, these findings are in close accordance with the model presented here and point to a conserved role for WTAs in preventing immune recognition of PG in both vertebrate and invertebrate hosts. The discovery of this phenomenon significantly deepens our understanding of the function of WTAs in S. aureus, adding them to the already impressive arsenal of immunoevasion mechanisms possessed by this important human pathogen.
These results also raise a number of follow-up questions deserving of further study. One informative direction would be to determine the specific structural components of WTA that mediate the polymer’s antibody-retardant properties. The highly charged ribitol-phosphate backbone of WTA likely plays a role, but its D-alanine and α- and β-O-N-acetyl-D-glucosamine (GlcNac) substituents may contribute significantly as well. Investigation into such questions should provide important insight into WTA’s emerging role in S. aureus pathogenesis and may also reveal novel pharmacologic targets for antistaphylococcal therapy. We believe that the rapidly expanding toolkit of synthetic cell wall probes, including those used here, will serve as a valuable adjunct to more traditional immunology and bacteriology techniques in these endeavors.23, 40, 41
EXPERIMENTAL SECTION
A full description of materials and methods may be found in the Supporting Information
Supplementary Material
Acknowledgments
Funding
This work was supported by a Camille and Henry Dreyfus Foundation New Faculty Award (to D.A.S.), a Novartis Early Career Award in Organic Chemistry (to D.A.S.), an Alfred P. Sloan Foundation Fellowship (BR2011-117 to D.A.S.), a National Institutes of Health New Innovator Award (1DP2OD002913-01 to D.A.S.), and a National Institutes of Health MSTP training grant (T32GM07205 to S.G.).
We gratefully acknowledge M.G. Pinho and D. Bartel’s helpful suggestions during the preparation of this manuscript.
ABBREVIATIONS
- FITC
fluorescein isothiocyanate
- PG
peptidoglycan
- WTA
wall teichoic acids
Footnotes
Notes
The authors declare the following competing financial interest(s): D.A.S. is a paid consultant for Bristol-Myers Squibb.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.5b00439.
Supporting figures and experimental methods (PDF) Three-dimensional SIM imaging (AVI)
References
- 1.Viner RM, Coffey C, Mathers C, Bloem P, Costello A, Santelli J, Patton GC. 50-year mortality trends in children and young people: a study of 50 low-income, middle-income, and high-income countries. Lancet. 2011;377:1162–1174. doi: 10.1016/S0140-6736(11)60106-2. [DOI] [PubMed] [Google Scholar]
- 2.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infectious disease clinics of North America. 2009;23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol. 2012;15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 5.Umeda A, Ueki Y, Amako K. Structure of the Staphylococcus aureus cell wall determined by the freeze-substitution method. J Bacteriol. 1987;169:2482–2487. doi: 10.1128/jb.169.6.2482-2487.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown S, Santa Maria JP, Jr, Walker S. Wall teichoic acids of gram-positive bacteria. Annu Rev Microbiol. 2013;67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qamar A, Golemi-Kotra D. Dual roles of FmtA in Staphylococcus aureus cell wall biosynthesis and autolysis. Antimicrob Agents Chemother. 2012;56:3797–3805. doi: 10.1128/AAC.00187-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, Yu W, Schwarz H, Peschel A, Gotz F. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol Microbiol. 2010;75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. [DOI] [PubMed] [Google Scholar]
- 9.Frankel MB, Schneewind O. Determinants of murein hydrolase targeting to cross-wall of Staphylococcus aureus peptidoglycan. J Biol Chem. 2012;287:10460–10471. doi: 10.1074/jbc.M111.336404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas R, Martinez RE, Gohring N, Schlag M, Josten M, Xia G, Hegler F, Gekeler C, Gleske AK, Gotz F, Sahl HG, Kappler A, Peschel A. Proton-binding capacity of Staphylococcus aureus wall teichoic acid and its role in controlling autolysin activity. PLoS One. 2012;7:e41415. doi: 10.1371/journal.pone.0041415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atilano ML, Pereira PM, Yates J, Reed P, Veiga H, Pinho MG, Filipe SR. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2010;107:18991–18996. doi: 10.1073/pnas.1004304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell J, Singh AK, Santa Maria JP, Jr, Kim Y, Brown S, Swoboda JG, Mylonakis E, Wilkinson BJ, Walker S. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem Biol. 2011;6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, Nicholson G, Neumeister B, Mond JJ, Peschel A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 14.Weidenmaier C, Kokai-Kun JF, Kulauzovic E, Kohler T, Thumm G, Stoll H, Gotz F, Peschel A. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int J Med Microbiol. 2008;298:505–513. doi: 10.1016/j.ijmm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Weidenmaier C, Peschel A, Xiong YQ, Kristian SA, Dietz K, Yeaman MR, Bayer AS. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J Infect Dis. 2005;191:1771–1777. doi: 10.1086/429692. [DOI] [PubMed] [Google Scholar]
- 16.Weidenmaier C, Peschel A, Kempf VA, Lucindo N, Yeaman MR, Bayer AS. DltABCD- and MprFmediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect Immun. 2005;73:8033–8038. doi: 10.1128/IAI.73.12.8033-8038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, Peschel A, Gotz F. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol. 2007;189:280–283. doi: 10.1128/JB.01221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler T, Weidenmaier C, Peschel A. Wall teichoic acid protects Staphylococcus aureus against antimicrobial fatty acids from human skin. J Bacteriol. 2009;191:4482–4484. doi: 10.1128/JB.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umeda A, Yokoyama S, Arizono T, Amako K. Location of peptidoglycan and teichoic acid on the cell wall surface of Staphylococcus aureus as determined by immunoelectron microscopy. J Electron Microscopy. 1992;41:46–52. [PubMed] [Google Scholar]
- 21.Sandhu S, Schouten JA, Thompson J, Davis M, Bugg TD. Detection of Staphylococcus aureus cell walls by enzymelinked immunoassay using antibodies prepared from a semi-synthetic peptidoglycan precursor. Analyst. 2012;137:1130–1136. doi: 10.1039/c2an16036f. [DOI] [PubMed] [Google Scholar]
- 22.Gautam S, Gniadek TJ, Kim T, Spiegel DA. Exterior design: strategies for redecorating the bacterial surface with small molecules. Trends Biotechnol. 2013;31:258–267. doi: 10.1016/j.tibtech.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson JW, Chamessian AG, McEnaney PJ, Murelli RP, Kazmierczak BI, Spiegel DA. A biosynthetic strategy for re-engineering the Staphylococcus aureus cell wall with non-native small molecules. ACS Chem Biol. 2010;5:1147–1155. doi: 10.1021/cb100195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marraffini LA, Dedent AC, Schneewind O. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol Mol Biol Rev. 2006;70:192–221. doi: 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindmark R, Thoren-Tolling K, Sjoquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J Immunol Methods. 1983;62:1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- 26.Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, Johnson MA, Ross JM, Hutchins JT, Patti JM. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect Immun. 2003;71:6864–6870. doi: 10.1128/IAI.71.12.6864-6870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbrugh HA, Peters R, Rozenberg-Arska M, Peterson PK, Verhoef J. Antibodies to cell wall peptidoglycan of Staphylococcus aureus in patients with serious staphylococcal infections. J Infect Dis. 1981;144:1–9. doi: 10.1093/infdis/144.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Sun D, Raisley B, Langer M, Iyer JK, Vedham V, Ballard JL, James JA, Metcalf J, Coggeshall KM. Antipeptidoglycan antibodies and Fcgamma receptors are the key mediators of inflammation in Gram-positive sepsis. J Immunol. 2012;189:2423–2431. doi: 10.4049/jimmunol.1201302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Gill CJ, Lee SH, Mann P, Zuck P, Meredith TC, Murgolo N, She X, Kales S, Liang L, Liu J, Wu J, Santa Maria J, Su J, Pan J, Hailey J, McGuinness D, Tan CM, Flattery A, Walker S, Black T, Roemer T. Discovery of wall teichoic acid inhibitors as potential anti-MRSA beta-lactam combination agents. Chem Biol. 2013;20:272–284. doi: 10.1016/j.chembiol.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristian SA, Lauth X, Nizet V, Goetz F, Neumeister B, Peschel A, Landmann R. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J Infect Dis. 2003;188:414–423. doi: 10.1086/376533. [DOI] [PubMed] [Google Scholar]
- 33.Jung DJ, An JH, Kurokawa K, Jung YC, Kim MJ, Aoyagi Y, Matsushita M, Takahashi S, Lee HS, Takahashi K, Lee BL. Specific serum Ig recognizing staphylococcal wall teichoic acid induces complement-mediated opsonophagocytosis against Staphylococcus aureus. J Immunol. 2012;189:4951–4959. doi: 10.4049/jimmunol.1201294. [DOI] [PubMed] [Google Scholar]
- 34.Park KH, Kurokawa K, Zheng L, Jung DJ, Tateishi K, Jin JO, Ha NC, Kang HJ, Matsushita M, Kwak JY, Takahashi K, Lee BL. Human serum mannose-binding lectin senses wall teichoic acid Glycopolymer of Staphylococcus aureus, which is restricted in infancy. J Biol Chem. 2010;285:27167–27175. doi: 10.1074/jbc.M110.141309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi K, Kurokawa K, Moyo P, Jung DJ, An JH, Chigweshe L, Paul E, Lee BL. Intradermal immunization with wall teichoic acid (WTA) elicits and augments an anti-WTA IgG response that protects mice from methicillinresistant Staphylococcus aureus infection independent of mannosebinding lectin status. PLoS One. 2013;8:e69739. doi: 10.1371/journal.pone.0069739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fura JM, Sabulski MJ, Pires MM. D-amino acid mediated recruitment of endogenous antibodies to bacterial surfaces. ACS Chem Biol. 2014;9:1480–1489. doi: 10.1021/cb5002685. [DOI] [PubMed] [Google Scholar]
- 37.An JH, Kurokawa K, Jung DJ, Kim MJ, Kim CH, Fujimoto Y, Fukase K, Coggeshall KM, Lee BL. Human SAP is a novel peptidoglycan recognition protein that induces complement-independent phagocytosis of Staphylococcus aureus. J Immunol. 2013;191:3319–3327. doi: 10.4049/jimmunol.1300940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atilano ML, Yates J, Glittenberg M, Filipe SR, Ligoxygakis P. Wall teichoic acids of Staphylococcus aureus limit recognition by the drosophila peptidoglycan recognition protein SA to promote pathogenicity. PLoS Pathog. 2011;7:e1002421. doi: 10.1371/journal.ppat.1002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atilano ML, Pereira PM, Vaz F, Catalao MJ, Reed P, Grilo IR, Sobral RG, Ligoxygakis P, Pinho MG, Filipe SR. Bacterial autolysins trim cell surface peptidoglycan to prevent detection by the Drosophila innate immune system. eLife. 2014;3:e02277. doi: 10.7554/eLife.02277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuru E, Hughes HV, Brown PJ, Hall E, Tekkam S, Cava F, de Pedro MA, Brun YV, Van Nieuwenhze MS. In Situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent D-amino acids. Angew Chem, Int Ed. 2012;51:12519–12523. doi: 10.1002/anie.201206749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gautam S, Kim T, Shoda T, Sen S, Deep D, Luthra R, Ferreira MT, Pinho MG, Spiegel DA. An activity based probe for studying crosslinking in live bacteria. Angew Chem, Int Ed. 2015;54:10492. doi: 10.1002/anie.201503869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.