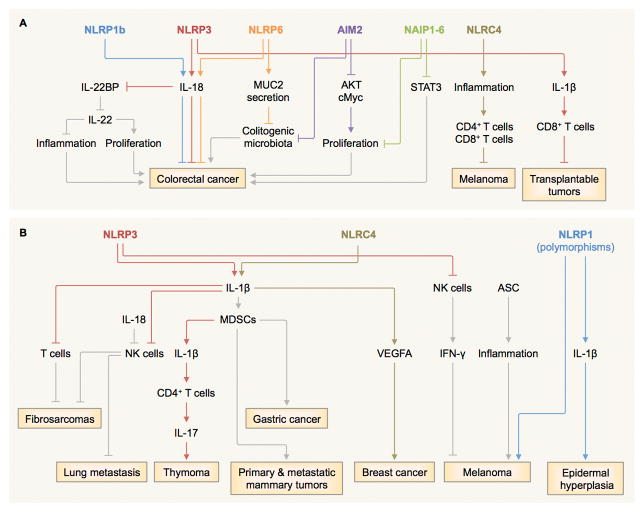
Figure 1. Diverse roles of inflammasome sensors in tumorigenesis.
A, NLRP1b, NLRP3 and NLRP6 mediate the production of IL-18, contributing to the protection against colitis-associated colorectal cancer (9–11,15,19,25–29). The IL-18 axis can also induce tumoricidal activity of NK cells against metastasized colonic tumor cells (19), downregulation IL-22 binding protein (IL-22BP) (20), and inhibiting the colonization of colitogenic microbiota (27) possibly through its role in MUC2 secretion by goblet cells (29,31). The NLRP3 inflammasome and the IL-1β–IL-1 receptor (IL-1R) signaling axis drives a T-cell response towards transplantable tumor cells (24). Mouse NAIP1–6 proteins control phosphorylation of STAT3 and the expression of genes encoding anti-apoptotic and proliferation-related molecules (32). NLRC4 controls the suppression of melanoma growth by amplifying inflammation in macrophages and potentiates production of IFN-γ in T cells (34). AIM2 inhibits phosphorylation of AKT and cMyc activities and stem cell proliferation, while preventing colonization of colitogenic microbiota (35,36). B, The NLRP3–IL-1β–IL-1R signaling axis suppresses the tumoricidal activity of NK cells and T cells and promotes methylcholanthrene (MCA)-induced fibrosarcomas (23). It also induces secretion of IL-17 by CD4+ T cells and dampens the anti-tumor efficacy of chemotherapeutic agents in thymoma (42). Overexpression of IL-1β mobilizes myeloid-derived suppressor cells (MDSCs) to the stomach and induces gastric cancer (40). IL-1 signaling drives accumulation of MDSCs and promotes primary and metastatic mammary tumors (41). Inflammasome-independent activity of NLRP3 suppresses NK cells and increases lung metastasis in certain models of melanoma (23,37). The NLRC4 inflammasome mediates expression of adipocyte-mediated vascular endothelial growth factor A (VEGFA) and accelerates the progression of breast cancer (43). In some cases, ASC increases the viability and growth of melanoma cells (38) and promotes inflammation in infiltrating myeloid cells and the development of skin cancer (39). Mutations in the gene encoding NLRP1 is linked to melanoma and epidermal hyperplasia in humans (5,6).