Abstract
Objective
We determined in patients with pulmonary arterial (PA) hypertension (PAH) whether in addition to increased production of elastase by PA smooth muscle cells (SMC) previously reported, PA elastic fibers are susceptible to degradation owing to their abnormal assembly.
Approach and Results
Fibrillin-1 and elastin are the major components of elastic fibers, and fibrillin-1 binds bone morphogenetic proteins (BMPs) and the large latent complex of transforming growth factor-β1 (TGFβ1). Thus, we considered whether BMPs like TGFβ1 contribute to elastic fiber assembly and whether this process is perturbed in PAH particularly when the BMP receptor, BMPR2, is mutant. We also assessed whether in mice with Bmpr2/1a compound heterozygosity, elastic fibers are susceptible to degradation.
In PA SMC and adventitial fibroblasts (PAF), TGFβ1 increased elastin mRNA but the elevation in elastin protein, was dependent on BMPR2; TGFβ1 and BMP4, via BMPR2, increased extracellular accumulation of fibrillin-1. Both BMP4- and TGFβ1-stimulated elastic fiber assembly were impaired in idiopathic (I) PAH-PAF vs. control cells, particularly those with hereditary (H) PAH and a BMPR2 mutation. This was related to profound reductions in elastin and fibrillin-1 mRNA. Elastin protein was increased in IPAH PAF by TGFβ1 but only minimally so in BMPR2 mutant cells. Fibrillin-1 protein increased only modestly in IPAH or HPAH PAF stimulated with BMP4 or TGFβ1. In Bmpr2/1a heterozygote mice, reduced PA fibrillin-1 was associated with elastic fiber susceptibility to degradation and more severe pulmonary hypertension.
Conclusion
Disrupting BMPR2 impairs TGFβ1 and BMP4 mediated elastic fiber assembly and is of pathophysiologic significance in PAH.
Keywords: fibrillin-1, pulmonary hypertension, extracellular matrix, vascular biology, growth factors and cytokines
Introduction
Pulmonary arterial hypertension (PAH) is a disease characterized by a progressive loss and obliteration of distal pulmonary arteries (PAs) that result in elevation of PA pressure, increased pulmonary vascular resistance to flow, right-sided heart failure and high morbidity as well as mortality. Developing effective treatments for PAH will require an approach that reverses the fundamental mechanisms causing the pulmonary vascular pathology.
Mutations resulting in loss of function of bone morphogenetic protein receptor 2 (BMPR2) occur in >70% of patients with familial PAH (FPAH) and in 25% of those with idiopathic PAH (IPAH), collectively denoted as hereditary or HPAH1, 2. Reduced expression of BMPR2 was shown in patients with IPAH without a mutation and even when PAH was related to other conditions (APAH)3. There is a low 20% penetrance of PAH in families carrying a BMPR2 mutation that has been partially addressed by recent genetic studies, indicating that affected vs. non-affected family members have reduced expression of BMPR2 from the normal allele4 or polymorphisms causing heightened transforming growth factor beta (TGFβ)1 signaling5. Other studies documented a reduction in the BMPR2 ligand, bone morphogenetic protein (BMP) 46. We have reported compensatory signaling pathways and gene expression in the unaffected BMPR2 mutation carriers7.
Elastic fibers provide elastic recoil to tissues such as the large arteries, lung, and skin. They consist of two major morphologically distinct components: elastin, a cross-linked polymer of tropoelastin, the monomeric secreted form of the protein8, and fibrillins, primarily fibrillin-1, a large (350 kDa), cysteine-rich glycoprotein9. Fibrillin microfibrils are the pivotal structures involved in storage and regulation of growth factors of the TGFβ superfamily, including TGFβ1 and BMPs10, 11. While the role of TGFβ signaling in the formation of elastic fibers in arteries as well as in other tissues is well known12, the contribution of BMPs to this process has not been studied.
Previous reports by our group have shown degradation of elastic fibers as a prominent feature of PAH, related to elevation in PA elastase activity identified as neutrophil elastase13. This enzyme is pivotal to vascular pathobiology since it releases mitogenic growth factors that are normally bound to intact elastic fibers14 and other matrix components. Degradation products of elastin, elastin peptides, are highly pro-inflammatory15, promoting the recruitment of activated inflammatory cells that produce cytokines and elastase that perpetuate adverse vascular remodeling. Mutations in the elastin gene (ELN) are associated with vascular disorders causing stenosis of pulmonary and systemic arteries in Willliams syndrome16, whereas mutations in the fibrillin-1 gene (FBN1) cause aneurysmal dilatation in Marfan syndrome17.
Given the physical association of BMPs and TGFβ with fibrillin-1, we hypothesized that these growth factors could play a complementary role in the organization and stability of the elastic fiber. Our study uncovered a co-dependence between BMPR2 and TGFβ in the assembly of the elastic fiber. TGFβ-stimulated production of the elastin protein was BMPR2-dependent, as was BMP-and TGFβ–mediated production of fibrillin-1. Hence elastic fiber assembly was impaired in IPAH vascular cells, particularly in those cells from patients with HPAH and a BMPR2 mutation. In transgenic mice with compound heterozygosity for Bmpr2/1a, that develop more severe pulmonary hypertension than wild type mice, we show production of a fibrillin-1 poor elastic fiber that is susceptible to elastase-mediated degradation. This is consistent with more advanced pathological features noted in HPAH patients with a BMPR2 mutation18 that have more rapid progression of disease19.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
TGFβ1 increases elastin mRNA and protein; TGFβ1 and BMP4 increase fibrillin-1 protein
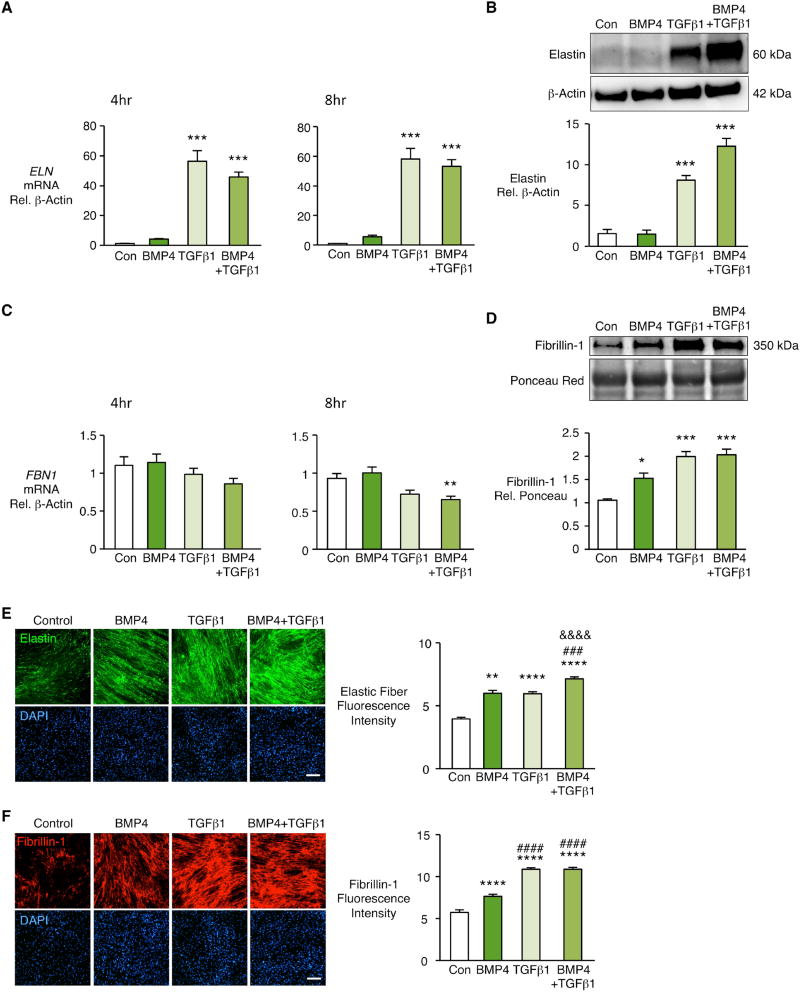
To investigate the relative roles of TGFβ1 and BMP4 signaling in the assembly of elastic fibers, we used human PA adventitial fibroblasts (PAF) and PA SMC at passages 3–6 cultured from donor control lungs from the Pulmonary Hypertension Breakthrough Initiative (see Materials and Methods). We found that TGFβ1 substantially increased ELN mRNA in PAF at 4h and 8h after stimulation (Fig. 1A) and elastin protein assessed at 48 h (Figure 1B). While BMP4 alone did not increase elastin mRNA or protein, it enhanced production of elastin protein when added to TGFβ1 (Figure 1B). Neither TGFβ1 nor BMP4 increased FBN1 mRNA levels in PAF (Fig. 1C). However, BMP4 and TGFβ1 increased fibrillin-1 protein in PAF conditioned media (Fig. 1D). There was, however no increase when the two were administered together. BMP4 was chosen as the ligand that best produced fibrillin-1 following pilot studies that also assessed BMP2 and BMP7 (data not shown). We could not account for the BMP4-mediated increase in fibrillin-1 that accumulated in the conditioned media on the basis of an increase in fibronectin previously described20 (Suppl. Fig. IA). We also investigated whether BMP4-mediated transcription of other elastin assembly proteins could promote accumulation of fibrillin-1, but saw no significant elevation in mRNA for emilin-1, lysyl oxidase, fibulin-5, and microfibrillar associated glycoprotein 1 or 2 (Suppl. Fig. IB). PAF were then stimulated with BMP4, TGFβ1 or both every other day for seven days to generate elastic fibers. We used both elastin (Figure 1E) and fibrillin-1 (Figure 1F) antibodies and applied quantitative morphometric techniques to images obtained by confocal microscopy to measure linear elastic immunoreactive fibers. Despite the fact that BMP4 did not increase elastin protein, we saw similar elastin immunoreactivity in the fibers when compared to TGFβ1. There was also an enhanced effect when the two were administered together. As with the biochemical data, BMP4 and TGFβ each resulted in fibrillin immunoreactivity in the deposited fibers but combining the two agents did not result in an increase in fibrillin. This suggested that BMP4 made efficient use of constitutive elastin to assemble elastic fibers.
Figure 1. TGFβ1 increases elastin mRNA and protein and TGFβ1 and BMP4 increase extracellular fibrillin-1 in human pulmonary artery fibroblasts.
Human pulmonary artery fibroblasts (PAF) isolated from unused donor lungs and used between passages 3–6 were stimulated with BMP4 (10ng/ml), TGFβ1 (2ng/ml), BMP4+TGFβ1, or vehicle (Con). (A, C) Fold change in mRNA of ELN and FBN1 was measured four and eight hours after stimulation. (B, D) Representative immunoblots above and densitometry below for elastin in cell lysates and fibrillin-1 in conditioned media 48h after stimulation. Beta-actin and Ponceau staining were used as loading controls for cell lysates and conditioned media respectively. Fibrillin-1 is normalized to Con. Bars represent Mean±SEM of n=3–5 independent experiments, *p<0.05, **p<0.01, ***p<0.001 vs. Con, by one-way ANOVA and post-hoc Bonferroni test.
(E, F) PAF of donor controls were seeded in glass chamber slides and grown for four days to confluence. Cells were starved overnight, then stimulated every other day for seven days with vehicle (Con), BMP4, TGFβ1 or BMP4+TGFβ1. Elastic fibers were visualized by indirect immunofluorescence of elastin (E) and fibrillin-1 (F). Right, fluorescence intensities quantified by Image J software and normalized to cell number assessed by nuclei DAPI staining. Scale bar=100μm. Bars represent Mean±SEM of n=4 independent experiments, **p<0.01, ****p<0.0001 vs. Con; ###p<0.001, ####p<0.0001 vs. BMP4; &&&&p<0.0001 vs. TGFβ1, by two-way ANOVA and post-hoc Bonferroni test.
In PA SMC as in PAF we observed an increase in elastin mRNA (Suppl. Fig. IIA) and protein (Suppl. Fig. IIB) in response to TGFβ1, but no increase with BMP4. As in PAF there was no increase with either agonist in fibrillin-1 mRNA in PA SMC (Suppl. Fig. IIC). However, only BMP4 increased fibrillin-1 protein in PA SMC (Suppl. Fig. IID). In contrast to PAF, PA SMC did not assemble elastic fibers in culture as assessed by confocal microscopy. To determine whether PA SMC were secreting a factor that interfered with elastic fiber assembly, we cultured PA SMC in fibroblast conditioned media and PAF in PA SMC conditioned media (Suppl. Fig. III A and B). In keeping with our hypothesis PA SMC assembled elastic fibers in response to BMP4 or TGFβ1 when cultured in PAF conditioned media whereas PAF cultured in PA SMC conditioned media only produced scant elastic fibers in response to TGFβ1.
We then applied 2-dimensional gel electrophoresis as described in the Methods to identify a secreted factor that suppressed elastic fiber assembly in PA SMC conditioned media that was not present in PAF conditioned media (Suppl. Fig. IIIC). Of the proteins differentially produced the proteoglycan decorin (DCN) (Suppl. Fig. IIID) was known to suppress elastin assembly 21 and it was reduced in the fibroblast relative to PA SMC conditioned media at baseline and with BMP4 or TGFβ1 stimulation. We then reduced decorin in PA SMC by siRNA and showed production of dense elastic fibers, (Suppl. Fig. IIIE) and we also added decorin to PAF conditioned media and suppressed elastic fiber formation (Suppl. Fig. IIIF).
TGFβ1 production of elastin is BMPR2-dependent
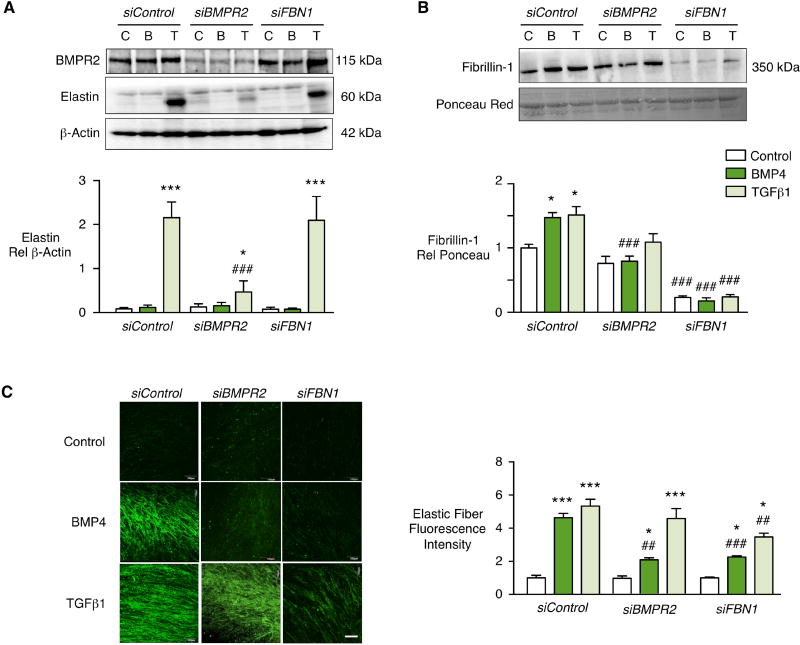
To determine the role of BMPR2 in PAF production of elastin and fibrillin-1, we reduced BMPR2 by greater than 60% using siRNA (Fig. 2A). Interestingly we found that TGFβ1 production of elastin protein (Figure 2A) was greatly reduced as was BMP4 and TGFβ1 production of fibrillin-1 (Fig. 2B). Neither the BMPR2-dependent increase in elastin in response to TGFβ1, nor the BMP4-mediated increase in fibrillin-1 could be explained by changes in downstream effectors pSMAD1/5 or p-p38, or in microRNAs previously implicated downstream of BMPR2 such as miR21 or miR2922, 23 (data not shown). Surprisingly, the TGFβ1 increase in elastin mRNA was preserved despite the loss of BMPR2 (Suppl. Fig. IV). This implied that BMPR2 may be necessary for TGFβ1 mediated translation of elastin mRNA or stability of the elastin protein. Loss of fibrillin-1 by siRNA did not interfere with elastin protein production in response to TGFβ1 (Figure 2A).
Figure 2. BMPR2 and fibrillin-1 are required for PAF elastic fiber formation.
PAF of donor controls were transfected with siRNA oligonucleotides targeting BMPR2 or FBN1, or with non-targeting siRNA (siControl). Starting 24h after transfection, the cells were stimulated for 48h with BMP4 (10ng/mL), TGFβ1 (2ng/mL) or vehicle (Control). (A, B) Representative immunoblot above and densitometry below for elastin and BMPR2 in cell lysates (A) and fibrillin-1 in conditioned media (B) of PAF donor controls. β-actin and Ponceau were used as loading controls for cell lysates and conditioned media respectively. (C) To assess elastic fibers cells were stimulated every other day for seven days and then visualized by indirect immunofluorescence of elastin and quantified by Image J software. Note that reduction of BMPR2 and fibrillin-1 inhibited BMP4-induced elastic fiber formation, and reduced fibrillin-1 also inhibited TGFβ1-induced fiber formation. The elastic fibers induced by TGFβ1 appear more fragmented in cells treated with BMPR2 siRNA. Scale bar=100µm. Bars represent Mean±SEM of n=3 independent experiments, *p<0.05, ***p<0.001 vs. unstimulated Control; ##p<0.01, ###p<0.001 vs. non-targeting siRNA (siControl) by two-way ANOVA and post-hoc Bonferroni test.
Non-targeting (control) siRNA did not affect the linear deposition of elastic fibers in response to BMP4 or TGFβ1 (Fig. 1C). As expected, the elastic fiber network produced following BMP4 stimulation was lost when BMPR2 was reduced by siRNA. While quantitatively the elastic fibers produced in response to TGFβ1 were not reduced with loss of BMPR2, they were more fragmented. This implies that the modest production of fibrillin and elastin in response to TGFβ1 was sufficient to allow for deposition of elastin fibers that were structurally abnormal. However, experiments with fibrillin siRNA indicated that both TGFβ1 and BMP4 require fibrillin-1 to deposit and assemble elastic fibers.
Fibroblasts from PAH patients have impaired elastic fiber assembly
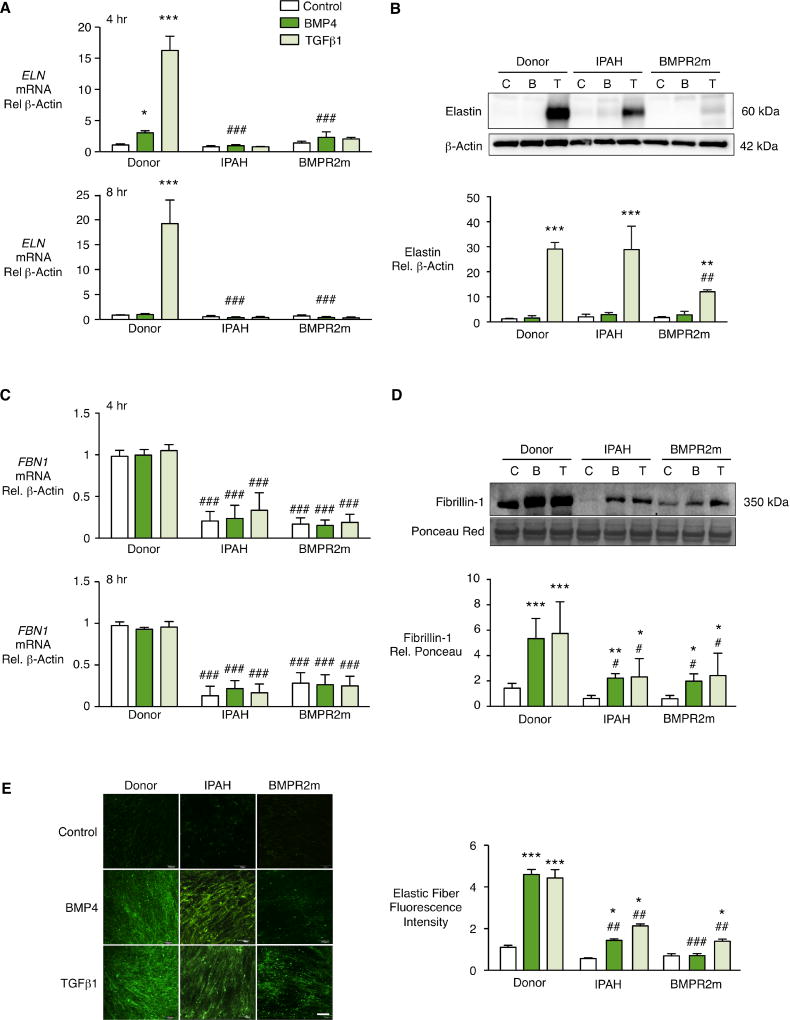
To investigate the consequence of BMPR2 dysfunction on elastic fiber formation in PAH, we compared PAF from unused donor lungs to PAF from lungs of patients with IPAH, and from those with a BMPR2 mutation related to HPAH. There was a profound decrease in ELN mRNA at baseline with no or minimal response to BMP4 or TGFβ1 in IPAH patients including the group with a BMPR2 mutation (Fig. 3A). TGFβ1-stimulated an increase in elastin protein in donor control and a more variable increase IPAH PAF, but there was a barely detectable increase in elastin protein in BMPR2 mutant cells (Fig. 3B). In PAF from patients with IPAH and in BMPR2 mutant cells, there was a profound reduction in FBN1 mRNA (Fig. 3C) that resulted in reduced fibrillin-1 protein in conditioned media in response to TGFβ1 or BMP4 (Fig. 3D). Thus in IPAH PAF, elastic fiber formation was greatly impaired in response to both BMP4 and TGFβ1, and in cells from the BMPR2 mutant group, it was barely detectable (Fig. 3E). This is consistent with the loss of fibrillin-1 (Suppl. Fig. V) as well as elastin protein in the latter group.
Figure 3. Reduced PAF elastic fibers from IPAH and HPAH with BMPR2 mutation (BMPR2m) related to elastin and fibrillin-1.
Elastic fiber formation by PAF from donor controls, IPAH and HPAH with a BMPR2 mutation (BMPR2m) analyzed as described in Figure 2. Fold change in ELN and FBN1 mRNA (A, C) measured four and eight hours following stimulation by BMP4 (10ng/ml), TGFβ1 (2ng/ml), or vehicle Control. Representative immunoblot and densitometry for elastin in the cell lysates and fibrillin-1 in the conditioned media (B, D) measured 48 hours after stimulation. (E) Elastic fibers were visualized by indirect immunofluorescence of elastin and quantified by Image J software. Scale bar=100µm. β–Actin and Ponceau were used as loading controls. Bars represent Mean±SEM of n=3 different cell lines (donor controls) per condition, *p<0.05, **p<0.001, ***p<0.001 vs. unstimulated (Control); #p<0.05, ##p<0.01, ###p<0.001 vs. Donor, by two-way ANOVA and post-hoc Bonferroni test.
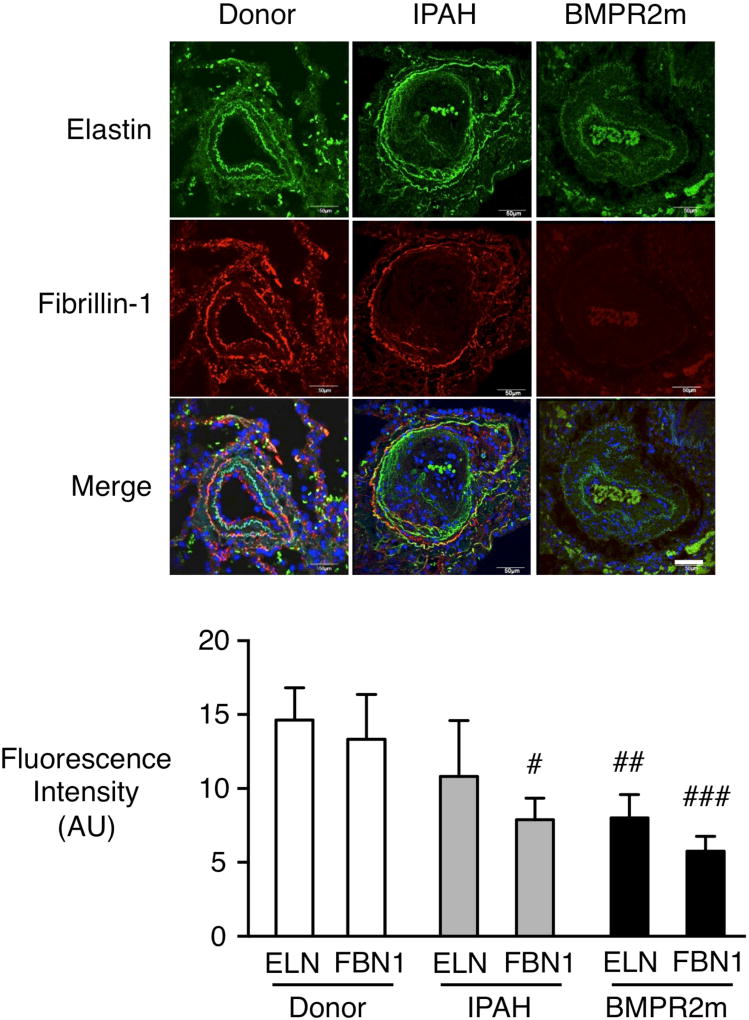
To relate our findings in cultured PAF to the tissue, we determined whether elastin and fibrillin-1 are also reduced in PAs of PAH patients. Immunofluorescence revealed reduced fibrillin-1 in IPAH PAs, particularly in the internal elastic lamina, and a loss of both elastin and fibrillin-1 in PAs in lungs from patients with HPAH and a BMPR2 mutation (Fig. 4).
Figure 4. Reduced PA elastin and fibrillin-1 in IPAH and HPAH pulmonary arteries with a BMPR2 mutation vs. donor control.
Representative PAs in lung tissue sections from donor control, IPAH and HPAH with a BMPR2 mutation (BMPR2m) were immunostained for elastin (green) and fibrillin-1 (red) and quantified below. Note that in PAs at the level of the terminal bronchiolus from IPAH lungs the elastic laminae are fragmented associated with a reduction in fibrillin-1, and in the BMPR2m there is substantial loss of elastic laminae associated with a decrease in both fibrillin-1 and elastin. Fluorescence intensity of elastin fibers was quantified by ImageJ. Bars represent Mean±SEM for donor control (n=5), IPAH (n=4) or BMPR2m (n=4). #p<0.05, ##p<0.01, ###p<0.001 vs. Donor by 2-way ANOVA and post-hoc Bonferroni test. Scale bar=50µm.
The PA elastic laminae of Bmpr2/1a compound heterozygote mice are susceptible to degradation and have more severe pulmonary hypertension
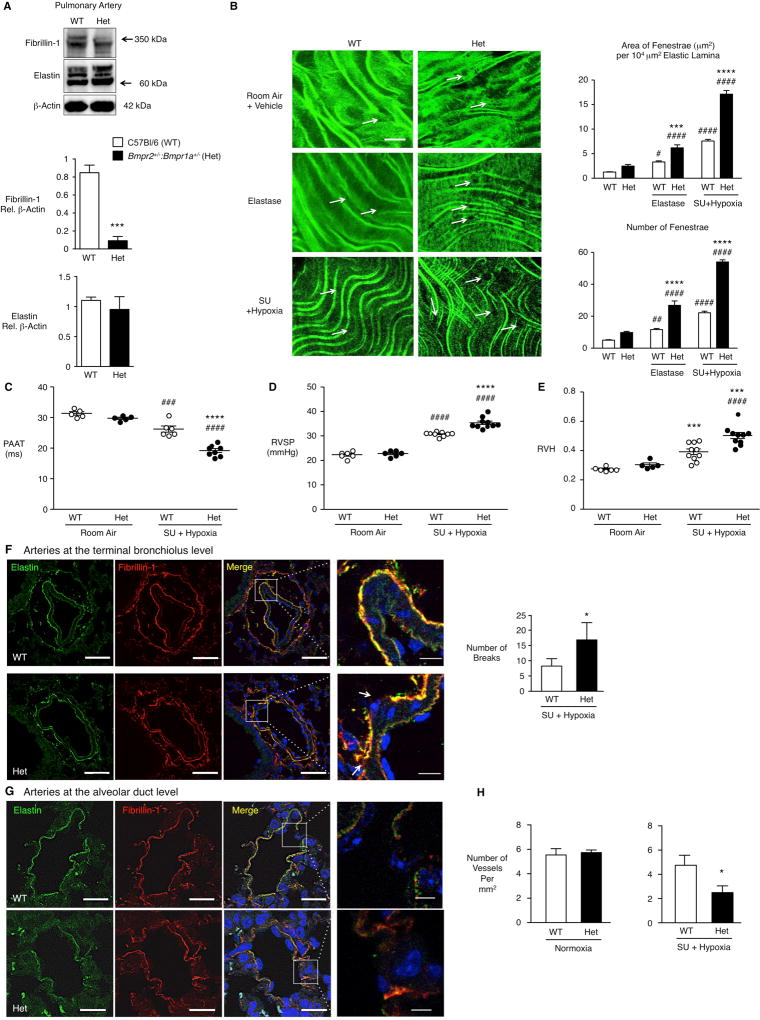
We hypothesized that mice with impaired function of BMPR2 would have poorly assembled elastic fibers that were susceptible to degradation. To test this, mice with compound heterozygosity for Bmpr2 and 1a (Bmpr2/1a) were used. We pooled three groups of central PAs from four mice/pool and documented a marked decrease in fibrillin-1 protein without a comparable reduction in elastin in the compound heterozygotes compared to wild type (WT) mice (Fig. 5A). Aortae exhibited a similar abnormality (data not shown).
Figure 5. Reduced fibrillin-1 and heightened susceptibility to elastic fiber degradation in Bmpr2/1a compound heterozygote mice.
(A) Representative immunoblot and densitometric analysis of elastin and fibrillin-1 of three sets of four pooled main PAs from Bmpr2/1a (Het) and wild type (WT) mice. Proteins were assessed on a reducing gel and the bands designated by the arrows were quantified. n=3 pools of Het or WT. (B) Representative confocal images of internal elastic lamina of central PAs from Het and WT mice. Central PAs were incubated with vehicle (PBS, top row) or porcine pancreatic elastase (5ng/mL, middle). On the bottom are PAs from mice exposed to Sugen 5416 (SU) and hypoxia as described in (C) below. Note the increase in size and number of fenestrations in the Het vs. WT PAs at baseline, following elastase treatment, or after Sugen and hypoxia (arrows). (SU+ hypoxia). On the right, quantification of number and area of fenestrations per total area of elastin assessed in six separate fields per condition (n=5/group). Scale bar=30µm.
(C–H) Het and WT mice were exposed to room air (normoxia), or 10% O2 (hypoxia) for three weeks following subcutaneous injection of VEGF receptor blocker Sugen 5416. (C) Pulmonary artery acceleration time (PAAT) measured as described in “Methods”. n=5–6 (WT) or 5–8 (Het) mice. (D, E) Development of PAH assessed by the right ventricular systolic pressure (RVSP, D) and right ventricular hypertrophy (RVH; E) given by the Fulton index (weight of RV/left ventricle and septum, RV/LV+S). Bars indicate Mean±SEM of n=5–6 mice for normoxia and n=9–10 mice for hypoxia. (F) Representative images of PAs at the level of the terminal bronchiolus stained for elastin and fibrillin-1. Note the increased number of breaks in the elastic lamina of Het vs. WT. Scale bars=50µm, 10µm in the higher magnification panels, right column. n=3. (G) Representative images of arteries at the alveolar duct level stained for elastin and fibrillin-1 from the het and WT mice exposed to hypoxia and Sugen. Scale bars=20µm, and 5µm in the higher magnification panels, right column. (H) Quantification of number of vessels per mm2 in normoxia and hypoxia. n=3.
Bars represent Mean±SEM. In A, F and H, *p<0.05, **p<0.01, ***p<0.001 vs. WT by t-test; In B, C, D, E, ***p<0.001 and ****p<0.0001, Het vs. WT; #p<0.05, ##p<0.01, ###p<0.001 and ####p<0.0001, SU+Hypoxia vs. Normoxia, same genotype, by two-way ANOVA and post-hoc Bonferroni test. In A, 3 sets of pooled PAs (n=4) from female (F) mice were used. For confocal measurements of elastin fenestrations, 3F and 2 males (M) in each genotype in each condition. PAAT, in RA, 3F, 2M of each genotype, and in Su+hypoxia 6M WT and 8M het. RVSP: in RA 3F and 3M of each genotype and in SU+/Hypoxia 9M WT and 10M hets. For RVH, 3M and 3F RA and 10M of each genotype in SU+Hypoxia.
We then evaluated whether the fibrillin-1 poor elastic fibers in those PAs from Bmpr2/1a heterozygotes relative to WT mice, were susceptible to degradation. As in our previous studies13 we perfused the vessels with either porcine pancreatic elastase or saline vehicle, and counted the number and size of fenestrations in the autofluorescent internal elastic lamina of the main PA by confocal microscopy. Both the area and number of fenestrations were increased twofold in the Bmpr2/1a heterozygote vs. WT elastic lamina following perfusion with elastase (Fig. 5B). We also determined whether a pathological insult resulting in pulmonary hypertension in association with an increase in lung elastase activity24, would result in greater degradation of elastin in Bmpr2/1 heterozygote vs. WT mice. Following administration of the VEGF receptor blocker Sugen 5416 by subcutaneous injection once a week during a three-week period of hypoxia (10% oxygen)25, we observed more pronounced degradation of PA elastic fibers in the Bmpr2/1 heterozygote vs. WT mice (Fig. 5B).
We determined whether the susceptibility of elastic fibers to degradation could be related to more severe pulmonary hypertension in the Bmpr2/1a vs. control mice. A decrease in pulmonary artery acceleration time in Bmpr2/1a heterozygotes when compared to WT controls (Fig. 5C) was associated with a small but significant increase in right ventricular systolic pressure (Fig. 5D) and with more severe right ventricular hypertrophy (Fig. 5E) following Sugen and hypoxia.
Immunofluorescence of elastin and fibrillin-1 in intrapulmonary arteries assessed at the level of the terminal respiratory unit showed fragmentation of elastic fibers in association with pulmonary hypertension, that was more severe in the Bmpr2/1a heterozygotes vs. WT mice (Fig. 5F, G). This was associated with a greater loss of alveolar wall and duct arteries relative to alveoli (Fig. 5H). Muscularization of distal vessels was similar (Suppl. Figure VI).
Discussion
While previous studies have focused on the antagonistic effects of BMPs and TGFβ in PAH5, our work indicates that, as in development26, both growth factors that co-occupy the same extracellular matrix protein, fibrillin-111, 27, take part in assembling elastic fibers. In this paper we show a previously unappreciated dependence of both TGFβ1 and BMP4 on BMPR2 in the assembly of the two main components of the elastic fiber, elastin and fibrillin-1 (Fig. 6). Neither the dependence on BMPR2 of TGFβ1-mediated production of elastin and fibrillin, nor the regulation of fibrillin-1 accumulation by BMPs had been previously established. In IPAH and HPAH BMPR2 mutant PAF, the profound reduction in ELN and FBN1 mRNA is related to the impaired production of elastic fibers. The reduction in PA fibrillin-1 in Bmpr2/1a heterozygote mice is associated with susceptibility of the elastic fibers to degradation, with more fragmented elastin, a greater loss of distal arteries and more severe pulmonary hypertension in response to VEGF receptor blockade and chronic hypoxia.
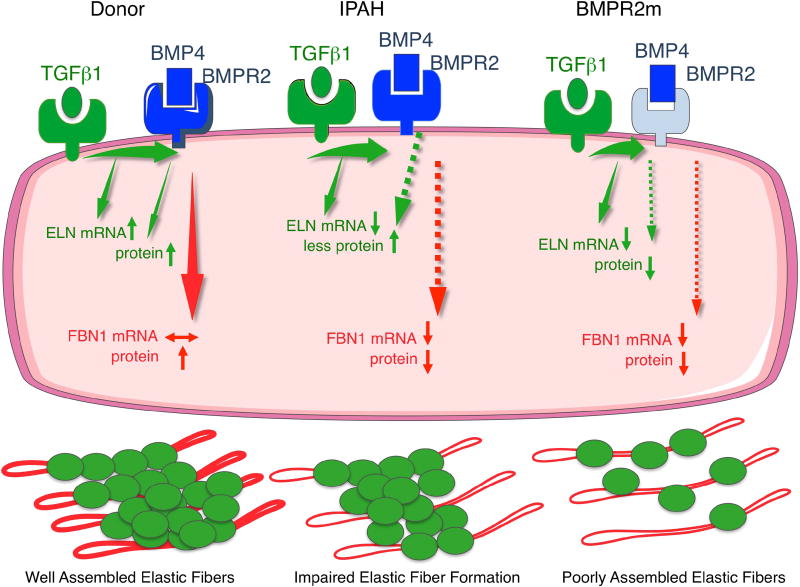
Figure 6.
Model of elastic fiber formation in control, IPAH and HPAH with a BMPR2 mutation.
While TGFβ1 stimulates elastin mRNA, production of elastin and fibrillin-1 proteins are both largely BMPR2 dependent. TGFβ1 and BMP4 via BMPR2 increase fibrillin-1. In IPAH the elastic fiber formation is impaired due to reduced fibrillin-1 mRNA and protein and elastin mRNA, although some elastin protein is produced in response to TGFβ1. When there is a mutation in BMPR2, fibrillin-1 and elastin protein are both markedly decreased in response to both TGFβ1 and BMP4, leading to poorly assembled elastic fibers.
Elastic laminae are an integral component of the PA and are fragmented in PAs of PAH patients in association with occlusive changes. Breakdown of elastic laminae is observed with neointimal formation that occurs with age28 and with vascular inflammation29, and yet little attention has been paid to whether the underlying composition of the elastic fibers might increase susceptibility to degradation. It is not surprising that TGFβ1 stimulated an increase in elastin mRNA and protein in both PA SMC and PAF, based upon previous studies30. It had also been shown that TGFβ stimulates production of fibrillin-131 although this was not studied in vascular cells.
Previous models of elastic fiber formation in culture used epithelial cells, dermal fibroblasts or rat smooth muscle cells32–35. For the present study, we developed a cell culture system that is robust in assessing factors that contribute to assembly of the elastic fibers in the vessel wall. Our studies revealed the importance of fibrillin-1 production for the formation of stable elastic fibers in culture. It is known that TGFβ1 stimulates elastin synthesis by a mechanism involving stabilization of the mRNA36. Loss of BMPR2 function likely impairs the stability of the elastin protein as the TGFβ1 mediated increase in elastin mRNA is preserved. The reduction in elastin protein may be related to metabolic changes associated with loss of BMPR237 that could perturb post-translational modifications necessary for elastin protein stability. This and the mechanism that explains the profound reduction in ELN and FBN1 mRNA in PAH cells will be of interest to pursue in future studies. Despite the failure of TGFβ1 to increase ELN mRNA in IPAH PAF, elastin protein was still increased. In BMPR2 mutant PAF, TGFβ could not increase elastin protein. Impaired stimulation of fibrillin-1 protein by both BMP4 and TGFβ1 may be largely related to the low mRNA levels in IPAH and BMPR2 mutant HPAH PAF. This is reflected in fibrillin-poor PAs in the tissue sections of IPAH patients. The reduction in elastin production in response to TGFβ1 that was prominent in PAH cells with a BMPR2 mutation is reflected in the more profound loss of elastin in elastic fibers in the PAs from these patients.
We found that the elastic fibers in the PAs of Bmpr2/1a compound heterozygote compared to WT mice are more susceptible to degradation by exogenous administration of elastase. It is the endogenous lung elastase associated with exposure to Sugen 5416 and chronic hypoxia24 that resulted in elastic fiber degradation in the PAs of both genotypes. However, the enhanced susceptibility to degradation observed in the Bmpr2/1a compound heterozygote compared to WT mice can be ascribed to the decrease in fibrillin-1. It was unexpected to find no reduction in elastin protein in the PAs of the Bmpr2/1a compound heterozygote compared to WT mice despite the fact that TGFβ1 is dependent on BMPR2 to produce elastic fibers. Perhaps reproducing this dependency in the mice requires greater deficiency of BMPR2 than haploinsufficiency, as was achieved with BMPR2 siRNA, or as is observed in the PAH subgroup with a BMPR2 mutation.
Despite the loss of fibrillin-1 and susceptibility of the elastic fiber to degradation, we did not see the aneurysmal dilatation observed when there is a mutation in FBN1, as in Marfan syndrome38. Recently SMC apoptosis has been described in transgenic mice with a mutation in FBN139 and loss of BMPR2 signaling by reduced BMPR2 can lead to resistance to apoptosis in SMC40. Bmpr2 heterozygous mice have no41 or little42 resting elevation of pulmonary arterial pressure under normal conditions or even with hypoxia alone41. We reasoned that compound heterozygosity might bring out a more consistent increase in hypoxia-induced pulmonary hypertension, but it was necessary to use an additional stimulus, i.e., VEGF receptor blockade. It is interesting that the pathological features associated with the more severe pulmonary hypertension related to a greater loss of distal arteries rather than to increased muscularization, but we anticipate that this feature, along with a greater increase in RVSP and RVH, may progress over time.
While heightened activity of TGFβ has been previously related to reduced activity of BMPR2 in pulmonary hypertension43, 44 our study shows a co-dependence between BMPR2 and TGFβ1 in assembling the key components of the elastic fiber. This reinforces the need for strategies that evaluate therapies for their ability to restore a balance between TGFβ and BMPR2 signaling.
Supplementary Material
Table 1.
Characteristics of Patients and Controls
| A. Controls (unused donor lungs)
| |||
|---|---|---|---|
| Study ID |
Age- Gender |
Cause of Death | Study |
| Don-1 | 60-F | Head Trauma Intracranial: Hemorrhage/Stroke; Death from Natural Causes | IF; WB; EF |
| Don-2 | 26-M | Gunshot wound to the head | qPCR; IF; WB; EF |
| Don-3 | 47-M | Head Trauma; Bicycle vs. Car Accident | qPCR; IF; WB; EF |
| Don-4 | 46-F | Cerebrovascular/Stroke; Intracranial Hemorrhage | qPCR; IF; WB |
| Don-5 | 25-M | Intracranial Hemorrhage | IF; WB |
| Don-6 | 56-F | Cerebrovascular Accident | IF; WB |
| Don-7 | 36-F | Subarachnoid hemorrhage | qPCR; WB |
| Don-8 | 1-M | Anoxia/Drowning | qPCR; WB |
| B. IPAH Patients
| |||||||
|---|---|---|---|---|---|---|---|
| ID | Age- Gender |
Known Mutation | PAP (s/d/m) |
PVR (WU) |
6MW (m) |
PAH Medications |
Study |
| IPAH-1 | 40-F | None | 62/24/36 | N/A | 233 | treprostinil ambrisentansildenafil | qPCR; IF; WB; EF |
| IPAH-2 | 40-M | None | 118/49/64 | 73 | 420 | sildenafil ambrisentantreprostinil | qPCR; IF; WB; EF |
| IPAH-3 | 51-M | None | 41/19/30 | 6.09 | 378 | sildenafil epoprostenol | qPCR; IF; WB; EF |
| IPAH-4 | 56-F | None | 83/39/57 | 11.41 | 137 | sildenafil ambrisentantreprostinil | IF; WB |
| IPAH-5 | 25-M | None | 65/15/36 | N/A | 511 | sildenafil epoprostenol treprostinil | IF; WB |
| IPAH-6 | 49-F | None | 100/50/75 | 16.76 | 326 | ambrisentan sildenafil epoprostenol | IF; WB |
| C. HPAH Patients with BMPR2 Mutation
| |||||||
|---|---|---|---|---|---|---|---|
| ID | Age- Gender |
Known Mutation | PAP (s/d/m) |
PVR (WU) |
6MW (m) |
PAH Medications |
Study |
| BMPR2m-1 | 27-F | BMPR2 c.76+5G>GA, probable mutation, not in dbSNP; may disrupt splicing SMAD9 – No | 110/49/69 | 12.11 | 421 | sildenafil, treprostinil bosentan iloprost | qPCR; IF; WB; EF |
| BMPR2m-2 | 33-F | BMPR2: c.961C>T; p.R321X, Nonsense, exon 7, c.961C>CT (nucleotide change), p.321R>R/X (amino acid change) SMAD9 – No | 87/29/48 | 9.74 | 288 | bosentan treprostinil sildenafil epoprostenol | qPCR; IF; WB; EF |
| BMPR2m-3 | 33-F | BMPR2: c.1471C>T; p.R491W, missense, exon 11, c.1471C>CT (nucleotide change), p.491R>R/W (amino acid change) SMAD9-No | 75/33/48 | 15.57 | 326 | epoprostenilbosentan sildenafil treprostinil | qPCR;IF; WB; EF |
| BMPR2m-4 | 37-M | BMPR2 c.1471C>T; p.R491W, missense, exon 11 | 119/51/77 | 14.22 | 308 | sildenafil, sitaxsentan, ambrisentanepoprostenol Imatinib (investigational medication) treprostinil | IF; WB; EF |
IPAH, idiopathic pulmonary arterial hypertension
HPAH, patients with familial pulmonary arterial hypertension classified separately because they were mutant for BMPR2 (Bmpr2m)
Hemodynamic data from catheterization was obtained from studies performed closest to transplantation. PAH medications are listed according to total drug exposure during treatment period of patient, not necessarily in combination.
PAP, pulmonary artery pressure (mmHg), s: systolic, d: diastolic, m: mean
PVR, pulmonary vascular resistance (dynes/sec•cm−5) (Baseline Fick PVR)
6MW, distance (m) walked in 6 minutes
NA, data not available
IF, Immunofluorescence
WB, protein expression assayed by western immunoblot
qPCR, gene expression assayed by qPCR
EF, fiber formation in culture
Highlights.
In pulmonary arterial smooth muscle cells and fibroblasts, TGFβ1 increases elastin mRNA and protein and fibrillin protein in a BMPR2 dependent manner and BMP4 also requires BMPR2 to increase extracellular fibrillin-1.
Elastic fiber assembly is impaired in pulmonary arterial fibroblasts from patients with IPAH, particularly when there is HPAH and BMPR2 mutation.
Greater degradation of elastic fibers is apparent in IPAH patient pulmonary arteries particularly in those with HPAH and a BMP2 mutation.
Mice with compound heterozygosity for Bmpr2/1a have fibrillin-1 poor elastic fibers that are susceptible to degradation by elastase or following exposure to VEGF receptor blockade and hypoxia.
Acknowledgments
Lung tissues from PAH patients and unused donor lungs were provided by the Pulmonary Hypertension Breakthrough Initiative (PHBI), which is funded by the NIH/NHLBI and by the Cardiovascular Medical Research and Education Fund (CMREF). The tissues were procured at the Transplant Procurement Centers at Allegheny Hospital (Pittsburgh, PA), Baylor University, The Cleveland Clinic, Stanford University, University of California–San Diego, Vanderbilt University, and the University of Alabama at Birmingham, and de-identified patient data were obtained via the Data Coordinating Center at the University of Michigan. The authors thank Dr. Michal Bental Roof for editorial assistance with preparation of the manuscript.
Sources of Funding
This project was supported by NIH/NHLBI grants R01 HL074186 and the Dunlevie Chair in Pediatric Cardiology at Stanford University (MR). The PHBI is funded by grants R24 HL123767 (M Geraci) and the Cardiovascular Medical Research and Education Fund (CMREF) Grant UL1RR024986. MAAA was supported by a Deutsche Forschungsgemeinschaft AL 1636 grant.
Footnotes
Disclosure
None of the co-authors have disclosures related to conflict of interest that influence the findings in this manuscript.
References
- 1.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Loyd JE, III, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nature Genetics. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 2.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene pph1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 4.Hamid R, Cogan JD, Hedges LK, Austin E, Phillips JA, 3rd, Newman JH, Loyd JE. Penetrance of pulmonary arterial hypertension is modulated by the expression of normal BMPR2 allele. Hum Mutat. 2009;30:649–654. doi: 10.1002/humu.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips JA, 3rd, Poling JS, Phillips CA, Stanton KC, Austin ED, Cogan JD, Wheeler L, Yu C, Newman JH, Dietz HC, Loyd JE. Synergistic heterozygosity for tgfbeta1 snps and BMPR2 mutations modulates the age at diagnosis and penetrance of familial pulmonary arterial hypertension. Genet Med. 2008;10:359–365. doi: 10.1097/GIM.0b013e318172dcdf. [DOI] [PubMed] [Google Scholar]
- 6.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: A gene microarray analysis. Circ Res. 2001;88:555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 7.Gu M, Shao NY, Sa S, et al. Patient-specific ipsc-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell. 2017;20:490–504. e495. doi: 10.1016/j.stem.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandberg LB, Zeikus RD, Coltrain IM. Tropoelastin purification from copper-deficient swine: A simplified method. Biochim Biophys Acta. 1971;236:542–545. doi: 10.1016/0005-2795(71)90237-6. [DOI] [PubMed] [Google Scholar]
- 9.Sakai LY, Keene DR, Glanville RW, Bachinger HP. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991;266:14763–14770. [PubMed] [Google Scholar]
- 10.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 11.Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem. 2008;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis EC, Rifkin DB. Dual functions for ltbp in lung development: Ltbp-4 independently modulates elastogenesis and TGF-beta activity. J Cell Physiol. 2009;219:14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YM, Haghighat L, Spiekerkoetter E, Sawada H, Alvira CM, Wang L, Acharya S, Rodriguez-Colon G, Orton A, Zhao M, Rabinovitch M. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am J Pathol. 2011;179:1560–1572. doi: 10.1016/j.ajpath.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson K, Rabinovitch M. Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J Cell Physiol. 1996;166:495–505. doi: 10.1002/(SICI)1097-4652(199603)166:3<495::AID-JCP4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, Stock AD, Leppert M, Keating MT. Hemizygosity at the elastin locus in a developmental disorder, williams syndrome. Nat Genet. 1993;5:11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- 17.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 18.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JD, Girerd B, Montani D, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: An individual participant data meta-analysis. Lancet Respir Med. 2016;4:129–137. doi: 10.1016/S2213-2600(15)00544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yabe Y, Hagiwara Y, Tsuchiya M, Honda M, Hatori K, Sonofuchi K, Kanazawa K, Koide M, Sekiguchi T, Itaya N, Itoi E. Decreased elastic fibers and increased proteoglycans in the ligamentum flavum of patients with lumbar spinal canal stenosis. J Orthop Res. 2016;34:1241–1247. doi: 10.1002/jor.23130. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Talati M, Fessel JP, et al. Estrogen metabolite 16alpha-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microrna-29-mediated modulation of cellular metabolism. Circulation. 2016;133:82–97. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parikh VN, Jin RC, Rabello S, et al. Microrna-21 integrates pathogenic signaling to control pulmonary hypertension: Results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickel NP, Spiekerkoetter E, Gu M, et al. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med. 2015;191:1273–1286. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes CJ, Im H, Cao A, et al. RNA sequencing analysis detection of a novel pathway of endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:356–366. doi: 10.1164/rccm.201408-1528OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by tgfbeta superfamily members. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez F, Rifkin DB. Extracellular microfibrils: Contextual platforms for tgfbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonicelli F, Bellon G, Debelle L, Hornebeck W. Elastin-elastases and inflamm-aging. Curr Top Dev Biol. 2007;79:99–155. doi: 10.1016/S0070-2153(06)79005-6. [DOI] [PubMed] [Google Scholar]
- 29.O'Blenes SB, Zaidi SH, Cheah AY, McIntyre B, Kaneda Y, Rabinovitch M. Gene transfer of the serine elastase inhibitor elafin protects against vein graft degeneration. Circulation. 2000;102:III289–295. doi: 10.1161/01.cir.102.suppl_3.iii-289. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Pierce RA, Wachi H, Mecham RP, Parks WC. An open reading frame element mediates posttranscriptional regulation of tropoelastin and responsiveness to transforming growth factor beta1. Mol Cell Biol. 1999;19:7314–7326. doi: 10.1128/mcb.19.11.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldwin AK, Cain SA, Lennon R, Godwin A, Merry CL, Kielty CM. Epithelial-mesenchymal status influences how cells deposit fibrillin microfibrils. J Cell Sci. 2014;127:158–171. doi: 10.1242/jcs.134270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robb BW, Wachi H, Schaub T, Mecham RP, Davis EC. Characterization of an in vitro model of elastic fiber assembly. Mol Biol Cell. 1999;10:3595–3605. doi: 10.1091/mbc.10.11.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinek A, Zhang S, Smith AC, Callahan JW. Impaired elastic-fiber assembly by fibroblasts from patients with either morquio b disease or infantile gm1-gangliosidosis is linked to deficiency in the 67-kd spliced variant of beta-galactosidase. Am J Hum Genet. 2000;67:23–36. doi: 10.1086/302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noda K, Dabovic B, Takagi K, et al. Latent TGF-beta binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proc Natl Acad Sci U S A. 2013;110:2852–2857. doi: 10.1073/pnas.1215779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370–377. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- 36.Kucich U, Rosenbloom JC, Abrams WR, Bashir MM, Rosenbloom J. Stabilization of elastin mRNA by TGF-beta: Initial characterization of signaling pathway. Am J Respir Cell Mol Biol. 1997;17:10–16. doi: 10.1165/ajrcmb.17.1.2816. [DOI] [PubMed] [Google Scholar]
- 37.Diebold I, Hennigs JK, Miyagawa K, et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015;21:596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eldadah ZA, Brenn T, Furthmayr H, Dietz HC. Expression of a mutant human fibrillin allele upon a normal human or murine genetic background recapitulates a marfan cellular phenotype. J Clin Invest. 1995;95:874–880. doi: 10.1172/JCI117737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, Tsao PS, Iosef C, Berry GJ, Mohr FW, Spin JM, Alvira CM, Robbins RC, Fischbein MP. miR-29b participates in early aneurysm development in marfan syndrome. Circ Res. 2012;110:312–324. doi: 10.1161/CIRCRESAHA.111.253740. [DOI] [PubMed] [Google Scholar]
- 40.Nasim MT, Ogo T, Chowdhury HM, Zhao L, Chen CN, Rhodes C, Trembath RC. BMPR-II deficiency elicits pro-proliferative and anti-apoptotic responses through the activation of tgfbeta-tak1-mapk pathways in PAH. Hum Mol Genet. 2012;21:2548–2558. doi: 10.1093/hmg/dds073. [DOI] [PubMed] [Google Scholar]
- 41.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98:818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- 42.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1241–1247. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 43.Upton PD, Davies RJ, Tajsic T, Morrell NW. Transforming growth factor-beta(1) represses bone morphogenetic protein-mediated smad signaling in pulmonary artery smooth muscle cells via smad3. Am J Respir Cell Mol Biol. 2013;49:1135–1145. doi: 10.1165/rcmb.2012-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbane AJ, Derrett-Smith E, Trinder SL, Good RB, Pearce A, Denton CP, Holmes AM. Impaired bone morphogenetic protein receptor II signaling in a transforming growth factor-beta-dependent mouse model of pulmonary hypertension and in systemic sclerosis. Am J Respir Crit Care Med. 2015;191:665–677. doi: 10.1164/rccm.201408-1464OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.